Protein p56 from the Bacillus subtilis phage phi29 inhibits DNA-binding ability of uracil-DNA glycosylase
- PMID: 17698500
- PMCID: PMC2018632
- DOI: 10.1093/nar/gkm584
Protein p56 from the Bacillus subtilis phage phi29 inhibits DNA-binding ability of uracil-DNA glycosylase
Abstract
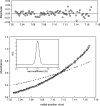
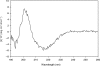
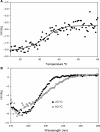
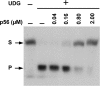
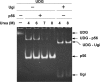
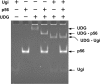
Protein p56 (56 amino acids) from the Bacillus subtilis phage 29 inactivates the host uracil-DNA glycosylase (UDG), an enzyme involved in the base excision repair pathway. At present, p56 is the only known example of a UDG inhibitor encoded by a non-uracil containing viral DNA. Using analytical ultracentrifugation methods, we found that protein p56 formed dimers at physiological concentrations. In addition, circular dichroism spectroscopic analyses revealed that protein p56 had a high content of beta-strands (around 40%). To understand the mechanism underlying UDG inhibition by p56, we carried out in vitro experiments using the Escherichia coli UDG enzyme. The highly acidic protein p56 was able to compete with DNA for binding to UDG. Moreover, the interaction between p56 and UDG blocked DNA binding by UDG. We also demonstrated that Ugi, a protein that interacts with the DNA-binding domain of UDG, was able to replace protein p56 previously bound to the UDG enzyme. These results suggest that protein p56 could be a novel naturally occurring DNA mimicry.
Figures


Similar articles
-
Characterization of Bacillus subtilis uracil-DNA glycosylase and its inhibition by phage φ29 protein p56.Mol Microbiol. 2011 Jun;80(6):1657-66. doi: 10.1111/j.1365-2958.2011.07675.x. Epub 2011 May 12. Mol Microbiol. 2011. PMID: 21542855
-
Protein mimicry of DNA from crystal structures of the uracil-DNA glycosylase inhibitor protein and its complex with Escherichia coli uracil-DNA glycosylase.J Mol Biol. 1999 Mar 26;287(2):331-46. doi: 10.1006/jmbi.1999.2605. J Mol Biol. 1999. PMID: 10080896
-
Phage phi29 protein p56 prevents viral DNA replication impairment caused by uracil excision activity of uracil-DNA glycosylase.Proc Natl Acad Sci U S A. 2008 Dec 9;105(49):19044-9. doi: 10.1073/pnas.0808797105. Epub 2008 Oct 9. Proc Natl Acad Sci U S A. 2008. PMID: 18845683 Free PMC article.
-
Role of host factors in bacteriophage φ29 DNA replication.Adv Virus Res. 2012;82:351-83. doi: 10.1016/B978-0-12-394621-8.00020-0. Adv Virus Res. 2012. PMID: 22420858 Review.
-
DNA bending and looping in the transcriptional control of bacteriophage phi29.FEMS Microbiol Rev. 2010 Sep;34(5):828-41. doi: 10.1111/j.1574-6976.2010.00219.x. Epub 2010 Mar 17. FEMS Microbiol Rev. 2010. PMID: 20412311 Review.
Cited by
-
DNA-Binding Proteins Essential for Protein-Primed Bacteriophage Φ29 DNA Replication.Front Mol Biosci. 2016 Aug 5;3:37. doi: 10.3389/fmolb.2016.00037. eCollection 2016. Front Mol Biosci. 2016. PMID: 27547754 Free PMC article. Review.
-
Architecturally diverse proteins converge on an analogous mechanism to inactivate Uracil-DNA glycosylase.Nucleic Acids Res. 2013 Oct;41(18):8760-75. doi: 10.1093/nar/gkt633. Epub 2013 Jul 26. Nucleic Acids Res. 2013. PMID: 23892286 Free PMC article.
-
Uracil-DNA glycosylases-structural and functional perspectives on an essential family of DNA repair enzymes.Protein Sci. 2014 Dec;23(12):1667-85. doi: 10.1002/pro.2554. Epub 2014 Oct 25. Protein Sci. 2014. PMID: 25252105 Free PMC article. Review.
-
Novel dimeric structure of phage φ29-encoded protein p56: insights into uracil-DNA glycosylase inhibition.Nucleic Acids Res. 2011 Dec;39(22):9779-88. doi: 10.1093/nar/gkr667. Epub 2011 Sep 2. Nucleic Acids Res. 2011. PMID: 21890898 Free PMC article.
-
A Processive Protein Chimera Introduces Mutations across Defined DNA Regions In Vivo.J Am Chem Soc. 2018 Sep 19;140(37):11560-11564. doi: 10.1021/jacs.8b04001. Epub 2018 Jul 18. J Am Chem Soc. 2018. PMID: 29991261 Free PMC article.
References
-
- Krokan HE, Drabløs F, Slupphaug G. Uracil in DNA – occurrence, consequences and repair. Oncogene. 2002;21:8935–8948. - PubMed
-
- Rada C, Di Noia JM, Neuberger MS. Mismatch recognition and uracil excision provide complementary paths to both Ig switching and the A/T-focused phase of somatic mutation. Mol. Cell. 2004;16:163–171. - PubMed
-
- Priet S, Sire J, Querat G. Uracils as a cellular weapon against viruses and mechanisms of viral escape. Curr. HIV Res. 2006;4:31–42. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

