Assessing disease severity in late infantile neuronal ceroid lipofuscinosis using quantitative MR diffusion-weighted imaging
- PMID: 17698521
- PMCID: PMC7977649
- DOI: 10.3174/ajnr.A0551
Assessing disease severity in late infantile neuronal ceroid lipofuscinosis using quantitative MR diffusion-weighted imaging
Abstract
Background and purpose: Late infantile neuronal ceroid lipofuscinosis (LINCL), a form of Batten disease, is a fatal neurodegenerative genetic disorder, diagnosed via DNA testing, that affects approximately 200 children in the United States at any one time. This study was conducted to evaluate whether quantitative data derived by diffusion-weighted MR imaging (DWI) techniques can supplement clinical disability scale information to provide a quantitative estimate of neurodegeneration, as well as disease progression and severity.
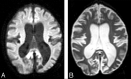
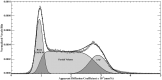
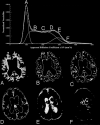
Materials and methods: This study prospectively analyzed 32 DWI examinations from 18 patients having confirmed LINCL at various stages of disease. A whole-brain apparent diffusion coefficient (ADC) histogram was fitted with a dual Gaussian function combined with a function designed to model voxels containing a partial volume fraction of brain parenchyma versus CSF. Previously published whole-brain ADC values of age-matched control subjects were compared with those of the LINCL patients. Correlations were tested between the peak ADC of the fitted histogram and patient age, disease severity, and a CNS disability scale adapted for LINCL.
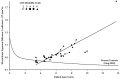
Results: ADC values assigned to brain parenchyma were higher than published ADC values for age-matched control subjects. ADC values between patients and control subjects began to differ at 5 years of age based on 95% confidence intervals. ADC values had a nearly equal correlation with patient age (R2=0.71) and disease duration (R2=0.68), whereas the correlation with the central nervous system disability scale (R2=0.27) was much weaker.
Conclusion: This study indicates that brain ADC values acquired using DWI may be used as an independent measure of disease severity and duration in LINCL.
Figures
Similar articles
-
Assessment of disease severity in late infantile neuronal ceroid lipofuscinosis using multiparametric MR imaging.AJNR Am J Neuroradiol. 2013 Apr;34(4):884-9. doi: 10.3174/ajnr.A3297. Epub 2012 Oct 4. AJNR Am J Neuroradiol. 2013. PMID: 23042927 Free PMC article.
-
Neurological deterioration in late infantile neuronal ceroid lipofuscinosis.Neurology. 2007 Aug 7;69(6):521-35. doi: 10.1212/01.wnl.0000267885.47092.40. Neurology. 2007. PMID: 17679671
-
Topological Alterations of the Structural Brain Connectivity Network in Children with Juvenile Neuronal Ceroid Lipofuscinosis.AJNR Am J Neuroradiol. 2019 Dec;40(12):2146-2153. doi: 10.3174/ajnr.A6306. Epub 2019 Nov 14. AJNR Am J Neuroradiol. 2019. PMID: 31727742 Free PMC article.
-
Homozygous missense TPP1 mutation associated with mild late infantile neuronal ceroid lipofuscinosis and the genotype-phenotype correlation.Seizure. 2019 Jul;69:180-185. doi: 10.1016/j.seizure.2018.08.027. Epub 2018 Sep 2. Seizure. 2019. PMID: 31059981
-
Diffusion-weighted MR of the brain: methodology and clinical application.Radiol Med. 2005 Mar;109(3):155-97. Radiol Med. 2005. PMID: 15775887 Review. English, Italian.
Cited by
-
Twenty-Year Survival Analysis of Adeno-Associated Virus Vector Serotype 2-Mediated Gene Therapy to the Central Nervous System for CLN2 Disease.Hum Gene Ther. 2024 Jan;36(1-2):28-35. doi: 10.1089/hum.2024.182. Epub 2025 Jan 2. Hum Gene Ther. 2024. PMID: 39745261
-
Therapeutic landscape for Batten disease: current treatments and future prospects.Nat Rev Neurol. 2019 Mar;15(3):161-178. doi: 10.1038/s41582-019-0138-8. Nat Rev Neurol. 2019. PMID: 30783219 Free PMC article. Review.
-
Assessment of disease severity in late infantile neuronal ceroid lipofuscinosis using multiparametric MR imaging.AJNR Am J Neuroradiol. 2013 Apr;34(4):884-9. doi: 10.3174/ajnr.A3297. Epub 2012 Oct 4. AJNR Am J Neuroradiol. 2013. PMID: 23042927 Free PMC article.
-
White matter changes in primary dystonia determined by 2D distribution analysis of diffusion tensor images.J Magn Reson Imaging. 2013 Jan;37(1):59-66. doi: 10.1002/jmri.23805. Epub 2012 Sep 17. J Magn Reson Imaging. 2013. PMID: 22987399 Free PMC article.
-
Gray Matter Growth Is Accompanied by Increasing Blood Flow and Decreasing Apparent Diffusion Coefficient during Childhood.AJNR Am J Neuroradiol. 2016 Sep;37(9):1738-44. doi: 10.3174/ajnr.A4772. Epub 2016 Apr 21. AJNR Am J Neuroradiol. 2016. PMID: 27102314 Free PMC article.
References
-
- Crystal RG, Sondhi D, Hackett NR, et al. Clinical protocol. Administration of a replication-deficient adeno-associated virus gene transfer vector expressing the human CLN2 cDNA to the brain of children with late infantile neuronal ceroid lipofuscinosis. Hum Gene Ther 2004;15:1131–54 - PubMed
-
- Autti T, Raininko R, Santavuori P, et al. MRI of neuronal ceroid lipofuscinosis. II. Postmortem MRI and histopathological study of the brain in 16 cases of neuronal ceroid lipofuscinosis of juvenile or late infantile type. Neuroradiology 1997;39:371–77 - PubMed
-
- Sondhi D, Hackett NR, Apblett RL, et al. Feasibility of gene therapy for late neuronal ceroid lipofuscinosis. Arch Neurol 2001;58:1793–98 - PubMed
-
- Steinfeld R, Heim P, von Gregory H, et al. Late infantile neuronal ceroid lipofuscinosis: quantitative description of the clinical course in patients with CLN2 mutations. Am J Med Genet 2002;112:347–54 - PubMed
-
- Ballon D, Dyke J, Schwartz LH, et al. Imaging therapeutic response in human bone marrow using rapid whole-body MRI. NMR Biomed 2000;13:321–28 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
