Species selectivity of mixed-lineage leukemia/trithorax and HCF proteolytic maturation pathways
- PMID: 17698583
- PMCID: PMC2168920
- DOI: 10.1128/MCB.00769-07
Species selectivity of mixed-lineage leukemia/trithorax and HCF proteolytic maturation pathways
Abstract
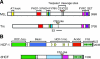
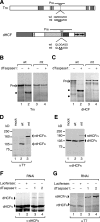
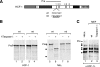
Site-specific proteolytic processing plays important roles in the regulation of cellular activities. The histone modification activity of the human trithorax group mixed-lineage leukemia (MLL) protein and the cell cycle regulatory activity of the cell proliferation factor herpes simplex virus host cell factor 1 (HCF-1) are stimulated by cleavage of precursors that generates stable heterodimeric complexes. MLL is processed by a protease called taspase 1, whereas the precise mechanisms of HCF-1 maturation are unclear, although they are known to depend on a series of sequence repeats called HCF-1(PRO) repeats. We demonstrate here that the Drosophila homologs of MLL and HCF-1, called Trithorax and dHCF, are both cleaved by Drosophila taspase 1. Although highly related, the human and Drosophila taspase 1 proteins display cognate species specificity. Thus, human taspase 1 preferentially cleaves MLL and Drosophila taspase 1 preferentially cleaves Trithorax, consistent with coevolution of taspase 1 and MLL/Trithorax proteins. HCF proteins display even greater species-specific divergence in processing: whereas dHCF is cleaved by the Drosophila taspase 1, human and mouse HCF-1 maturation is taspase 1 independent. Instead, human and Xenopus HCF-1PRO repeats are cleaved in vitro by a human proteolytic activity with novel properties. Thus, from insects to humans, HCF proteins have conserved proteolytic maturation but evolved different mechanisms.
Figures


References
-
- Ayton, P. M., and M. L. Cleary. 2001. Molecular mechanisms of leukemogenesis mediated by MLL fusion proteins. Oncogene 20:5695-5707. - PubMed
-
- Breen, T. R., and P. J. Harte. 1991. Molecular characterization of the trithorax gene, a positive regulator of homeotic gene expression in Drosophila. Mech. Dev. 35:113-127. - PubMed
-
- Brown, M. S., and J. L. Goldstein. 1997. The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell 89:331-340. - PubMed
-
- Djabali, M., L. Selleri, P. Parry, M. Bower, B. D. Young, and G. A. Evans. 1992. A trithorax-like gene is interrupted by chromosome 11q23 translocations in acute leukaemias. Nat. Genet. 2:113-118. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
