Human Cdc34 employs distinct sites to coordinate attachment of ubiquitin to a substrate and assembly of polyubiquitin chains
- PMID: 17698585
- PMCID: PMC2168909
- DOI: 10.1128/MCB.00812-07
Human Cdc34 employs distinct sites to coordinate attachment of ubiquitin to a substrate and assembly of polyubiquitin chains
Abstract
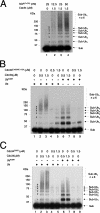
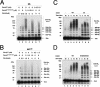
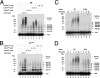
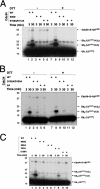
The Cdc34 E2 ubiquitin (Ub) conjugating enzyme catalyzes polyubiquitination of a substrate recruited by the Skp1-Cullin 1-F-box protein-ROC1 E3 Ub ligase. Using mutagenesis studies, we now show that human Cdc34 employs distinct sites to coordinate the transfer of Ub to a substrate and the assembly of polyubiquitin chains. Mutational disruption of the conserved charged stretch (residues 143 to 153) or the acidic loop residues D102 and D103 led to accumulation of monoubiquitinated IkappaBalpha while failing to yield polyubiquitin chains, due to a catalytic defect in Ub-Ub ligation. These results suggest an ability of human Cdc34 to position the attacking Ub for assembly of polyubiquitin chains. Analysis of Cdc34N85Q and Cdc34S138A revealed severe defects of these mutants in both poly- and monoubiquitination of IkappaBalpha, supporting a role for N85 in stabilizing the oxyanion and in coordinating, along with S138, the attacking lysine for catalysis. Finally, Cdc34S95D and Cdc34(E108A/E112A) abolished both poly- and monoubiquitination of IkappaBalpha. Unexpectedly, the catalytic defects of these mutants in di-Ub synthesis can be rescued by fusion of a glutathione S-transferase moiety at E2's N terminus. These findings support the hypothesis that human Cdc34 S95 and E108/E112 are required to position the donor Ub optimally for catalysis, in a manner that might depend on E2 dimerization.
Figures



Similar articles
-
Molecular basis for lysine specificity in the yeast ubiquitin-conjugating enzyme Cdc34.Mol Cell Biol. 2010 May;30(10):2316-29. doi: 10.1128/MCB.01094-09. Epub 2010 Mar 1. Mol Cell Biol. 2010. PMID: 20194622 Free PMC article.
-
Proximity-induced activation of human Cdc34 through heterologous dimerization.Proc Natl Acad Sci U S A. 2005 Oct 18;102(42):15053-8. doi: 10.1073/pnas.0507646102. Epub 2005 Oct 6. Proc Natl Acad Sci U S A. 2005. PMID: 16210246 Free PMC article.
-
Association of the disordered C-terminus of CDC34 with a catalytically bound ubiquitin.J Mol Biol. 2011 Apr 1;407(3):425-38. doi: 10.1016/j.jmb.2011.01.047. Epub 2011 Feb 4. J Mol Biol. 2011. PMID: 21296085
-
Protein monoubiquitination and polyubiquitination generate structural diversity to control distinct biological processes.IUBMB Life. 2012 Feb;64(2):136-42. doi: 10.1002/iub.589. Epub 2011 Nov 30. IUBMB Life. 2012. PMID: 22131221 Review.
-
Orchestra for assembly and fate of polyubiquitin chains.Essays Biochem. 2005;41:1-14. doi: 10.1042/EB0410001. Essays Biochem. 2005. PMID: 16250894 Review.
Cited by
-
Molecular basis for lysine specificity in the yeast ubiquitin-conjugating enzyme Cdc34.Mol Cell Biol. 2010 May;30(10):2316-29. doi: 10.1128/MCB.01094-09. Epub 2010 Mar 1. Mol Cell Biol. 2010. PMID: 20194622 Free PMC article.
-
Enzymatic Logic of Ubiquitin Chain Assembly.Front Physiol. 2019 Jul 5;10:835. doi: 10.3389/fphys.2019.00835. eCollection 2019. Front Physiol. 2019. PMID: 31333493 Free PMC article. Review.
-
The human Cdc34 carboxyl terminus contains a non-covalent ubiquitin binding activity that contributes to SCF-dependent ubiquitination.J Biol Chem. 2010 Jun 4;285(23):17754-62. doi: 10.1074/jbc.M109.090621. Epub 2010 Mar 30. J Biol Chem. 2010. PMID: 20353940 Free PMC article.
-
The ubiquitin-conjugating enzyme CDC34 is essential for cytokinesis in contrast to putative subunits of a SCF complex in Trypanosoma brucei.PLoS Negl Trop Dis. 2017 Jun 13;11(6):e0005626. doi: 10.1371/journal.pntd.0005626. eCollection 2017 Jun. PLoS Negl Trop Dis. 2017. PMID: 28609481 Free PMC article.
-
Solution structure and dynamics of human ubiquitin conjugating enzyme Ube2g2.Proteins. 2010 Apr;78(5):1291-301. doi: 10.1002/prot.22648. Proteins. 2010. PMID: 20014027 Free PMC article.
References
-
- Chen, A., K. Wu, S. Y. Fuchs, P. Tan, C. Gomez, and Z.-Q. Pan. 2000. The conserved RING-H2 finger of ROC1 is required for ubiquitin ligation. J. Biol. Chem. 275:15432-15439. - PubMed
-
- Deffenbaugh, A. E., K. M. Scaglione, L. Zhang, J. M. Moore, T. Buranda, L. A. Sklar, and D. Skowyra. 2003. Release of ubiquitin-charged Cdc34-S-Ub from the RING domain is essential for ubiquitination of the SCF(Cdc4)-bound substrate Sic1. Cell 114:611-622. - PubMed
-
- Feldman, R. M., C. C. Correll, K. B. Kaplan, and R. J. Deshaies. 1997. A complex of Cdc4p, Skp1p, and Cdc53p/cullin catalyzes ubiquitination of the phosphorylated CDK inhibitor Sic1p. Cell 91:221-230. - PubMed
-
- Goebl, M. G., J. Yochem, S. Jentsch, J. P. McGrath, A. Varshavsky, and B. Byers. 1988. The yeast cell cycle gene CDC34 encodes a ubiquitin-conjugating enzyme. Science 241:1331-1335. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
