Control of inward rectifier K channel activity by lipid tethering of cytoplasmic domains
- PMID: 17698595
- PMCID: PMC2151642
- DOI: 10.1085/jgp.200709764
Control of inward rectifier K channel activity by lipid tethering of cytoplasmic domains
Abstract
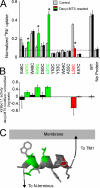
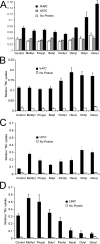
Interactions between nontransmembrane domains and the lipid membrane are proposed to modulate activity of many ion channels. In Kir channels, the so-called "slide-helix" is proposed to interact with the lipid headgroups and control channel gating. We examined this possibility directly in a cell-free system consisting of KirBac1.1 reconstituted into pure lipid vesicles. Cysteine substitution of positively charged slide-helix residues (R49C and K57C) leads to loss of channel activity that is rescued by in situ restoration of charge following modification by MTSET(+) or MTSEA(+), but not MTSES(-) or neutral MMTS. Strikingly, activity is also rescued by modification with long-chain alkyl-MTS reagents. Such reagents are expected to partition into, and hence tether the side chain to, the membrane. Systematic scanning reveals additional slide-helix residues that are activated or inhibited following alkyl-MTS modification. A pattern emerges whereby lipid tethering of the N terminus, or C terminus, of the slide-helix, respectively inhibits, or activates, channel activity. This study establishes a critical role of the slide-helix in Kir channel gating, and directly demonstrates that physical interaction of soluble domains with the membrane can control ion channel activity.
Figures




Similar articles
-
Conformational changes at cytoplasmic intersubunit interactions control Kir channel gating.J Biol Chem. 2017 Jun 16;292(24):10087-10096. doi: 10.1074/jbc.M117.785154. Epub 2017 Apr 26. J Biol Chem. 2017. PMID: 28446610 Free PMC article.
-
Flexibility of the Kir6.2 inward rectifier K(+) channel pore.Proc Natl Acad Sci U S A. 2001 Mar 27;98(7):4227-32. doi: 10.1073/pnas.061452698. Epub 2001 Mar 6. Proc Natl Acad Sci U S A. 2001. PMID: 11274446 Free PMC article.
-
Crystal structure of the potassium channel KirBac1.1 in the closed state.Science. 2003 Jun 20;300(5627):1922-6. doi: 10.1126/science.1085028. Epub 2003 May 8. Science. 2003. PMID: 12738871
-
Gating of inward rectifier K(+) channels by proton-mediated interactions of intracellular protein domains.Trends Cardiovasc Med. 2002 Jan;12(1):5-13. doi: 10.1016/s1050-1738(01)00132-3. Trends Cardiovasc Med. 2002. PMID: 11796238 Review.
-
Lipids driving protein structure? Evolutionary adaptations in Kir channels.Channels (Austin). 2010 May-Jun;4(3):139-41. doi: 10.4161/chan.4.3.12129. Epub 2010 May 1. Channels (Austin). 2010. PMID: 21150302 Free PMC article. Review.
Cited by
-
New Structural insights into Kir channel gating from molecular simulations, HDX-MS and functional studies.Sci Rep. 2020 May 21;10(1):8392. doi: 10.1038/s41598-020-65246-z. Sci Rep. 2020. PMID: 32439887 Free PMC article.
-
DEND mutation in Kir6.2 (KCNJ11) reveals a flexible N-terminal region critical for ATP-sensing of the KATP channel.Biophys J. 2008 Nov 15;95(10):4689-97. doi: 10.1529/biophysj.108.138685. Epub 2008 Aug 15. Biophys J. 2008. PMID: 18708460 Free PMC article.
-
The C-terminal PAL motif and transmembrane domain 9 of presenilin 1 are involved in the formation of the catalytic pore of the gamma-secretase.J Neurosci. 2008 Jun 11;28(24):6264-71. doi: 10.1523/JNEUROSCI.1163-08.2008. J Neurosci. 2008. PMID: 18550769 Free PMC article.
-
Inwardly rectifying potassium channels: Critical insights for insect species and Apis mellifera.Channels (Austin). 2025 Dec;19(1):2529250. doi: 10.1080/19336950.2025.2529250. Epub 2025 Jul 10. Channels (Austin). 2025. PMID: 40641062 Free PMC article. Review.
-
Novel compounds that specifically bind and modulate MscL: insights into channel gating mechanisms.FASEB J. 2019 Mar;33(3):3180-3189. doi: 10.1096/fj.201801628R. Epub 2018 Oct 25. FASEB J. 2019. PMID: 30359098 Free PMC article.
References
-
- Baukrowitz, T., U. Schulte, D. Oliver, S. Herlitze, T. Krauter, S.J. Tucker, J.P. Ruppersberg, and B. Fakler. 1998. PIP2 and PIP as determinants for ATP inhibition of KATP channels. Science. 282:1141–1144. - PubMed
-
- Boccuni, P., L. Del Vecchio, R. Di Noto, and B. Rotoli. 2000. Glycosyl phosphatidylinositol (GPI)-anchored molecules and the pathogenesis of paroxysmal nocturnal hemoglobinuria. Crit. Rev. Oncol. Hematol. 33:25–43. - PubMed
-
- Enkvetchakul, D., I. Jeliazkova, and C.G. Nichols. 2005. Direct modulation of Kir channel gating by membrane phosphatidylinositol 4,5-bisphosphate. J. Biol. Chem. 280:35785–35788. - PubMed

