KCNE1 and KCNE3 stabilize and/or slow voltage sensing S4 segment of KCNQ1 channel
- PMID: 17698596
- PMCID: PMC2151641
- DOI: 10.1085/jgp.200709805
KCNE1 and KCNE3 stabilize and/or slow voltage sensing S4 segment of KCNQ1 channel
Abstract
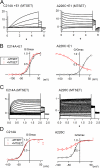
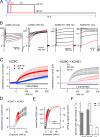
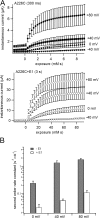
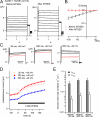
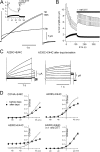
KCNQ1 is a voltage-dependent K(+) channel whose gating properties are dramatically altered by association with auxiliary KCNE proteins. For example, KCNE1, which is mainly expressed in heart and inner ear, markedly slows the activation kinetics of KCNQ1. Whether the voltage-sensing S4 segment moves differently in the presence of KCNE1 is not yet known, however. To address that question, we systematically introduced cysteine mutations, one at a time, into the first half of the S4 segment of human KCNQ1. A226C was found out as the most suited mutant for a methanethiosulfonate (MTS) accessibility analysis because it is located at the N-terminal end of S4 segment and its current was stable with repetitive stimuli in the absence of MTS reagent. MTS accessibility analysis revealed that the apparent second order rate constant for modification of the A226C mutant was state dependent, with faster modification during depolarization, and was 13 times slower in the presence of KCNE1 than in its absence. In the presence of KCNE3, on the other hand, the second order rate constant for modification was not state dependent, indicating that the C226 residue was always exposed to the extracellular milieu, even at the resting membrane potential. Taken together, these results suggest that KCNE1 stabilizes the S4 segment in the resting state and slows the rate of transition to the active state, while KCNE3 stabilizes the S4 segment in the active state. These results offer new insight into the mechanism of KCNQ1 channel modulation by KCNE1 and KCNE3.
Figures

References
-
- Abbott, G.W., F. Sesti, I. Splawski, M.E. Buck, M.H. Lehmann, K.W. Timothy, M.T. Keating, and S.A. Goldstein. 1999. MiRP1 forms IKr potassium channels with HERG and is associated with cardiac arrhythmia. Cell. 97:175–187. - PubMed
-
- Abbott, G.W., M.H. Butler, S. Bendahhou, M.C. Dalakas, L.J. Ptacek, and S.A. Goldstein. 2001. MiRP2 forms potassium channels in skeletal muscle with Kv3.4 and is associated with periodic paralysis. Cell. 104:217–231. - PubMed
-
- Akabas, M.H., D.A. Stauffer, M. Xu, and A. Karlin. 1992. Acetylcholine receptor channel structure probed in cysteine-substitution mutants. Science. 258:307–310. - PubMed
-
- Barhanin, J., F. Lesage, E. Guillemare, M. Fink, M. Lazdunski, and G. Romey. 1996. KVLQT1 and lsK (minK) proteins associate to form the IKs cardiac potassium current. Nature. 384:78–80. - PubMed

