A cysteine protease inhibitor cures Chagas' disease in an immunodeficient-mouse model of infection
- PMID: 17698625
- PMCID: PMC2151429
- DOI: 10.1128/AAC.00436-07
A cysteine protease inhibitor cures Chagas' disease in an immunodeficient-mouse model of infection
Abstract
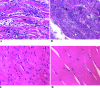
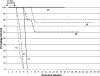
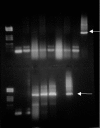
Chagas' disease, caused by the parasite Trypanosoma cruzi, remains the leading cause of cardiopathy in Latin America with about 12 million people infected. Classic clinical manifestations derive from infection of muscle cells leading to progressive cardiomyopathy, while some patients develop megacolon or megaesophagus. A very aggressive clinical course including fulminant meningoencephalitis has been reported in patients who contract Chagas' disease in the background of immunodeficiency. This includes patients with human immunodeficiency virus infection as well as patients receiving immunosuppressive therapy for organ transplant. Currently, only two drugs are approved for the treatment of Chagas' disease, nifurtimox and benznidazole. Both have significant limitations due to common and serious side effects as well as limited availability. A promising group of new drug leads for Chagas' disease is cysteine protease inhibitors targeting cruzain, the major protease of T. cruzi. The inhibitor N-methyl-Pip-F-homoF-vinyl sulfonyl phenyl (N-methyl-Pip-F-hF-VS phi) is in late-stage preclinical development. Therefore, the question arose as to whether protease inhibitors targeting cruzain would have efficacy in Chagas' disease occurring in the background of immunodeficiency. To address this question, we studied the course of infection in recombinase-deficient (Rag1(-/-)) and normal mice infected with T. cruzi. Infections localized to heart and skeletal muscle in untreated normal animals, while untreated Rag1(-/-) mice showed severe infection in all organs and predominantly in liver and spleen. Treatment with the dipeptide N-methyl-Pip-F-hF-VS phi rescued immunodeficient animals from lethal Chagas' infection. The majority (60 to 100%) of inhibitor-treated Rag1(-/-) mice had increased survival, negative PCR, and normal tissues by histopathological examination.
Figures



Similar articles
-
In vitro and in vivo studies of the trypanocidal properties of WRR-483 against Trypanosoma cruzi.PLoS Negl Trop Dis. 2010 Sep 14;4(9):e825. doi: 10.1371/journal.pntd.0000825. PLoS Negl Trop Dis. 2010. PMID: 20856868 Free PMC article.
-
Novel cruzain inhibitors for the treatment of Chagas' disease.Chem Biol Drug Des. 2012 Sep;80(3):398-405. doi: 10.1111/j.1747-0285.2012.01416.x. Epub 2012 Jun 27. Chem Biol Drug Des. 2012. PMID: 22613098 Free PMC article.
-
[Trypanocidal effect of cysteine protease inhibitors in vitro and in vivo in experimental Chagas disease].Medicina (B Aires). 1999;59 Suppl 2:171-5. Medicina (B Aires). 1999. PMID: 10668260 Review. Spanish.
-
Anti-parasitic effect of vitamin C alone and in combination with benznidazole against Trypanosoma cruzi.PLoS Negl Trop Dis. 2018 Sep 21;12(9):e0006764. doi: 10.1371/journal.pntd.0006764. eCollection 2018 Sep. PLoS Negl Trop Dis. 2018. PMID: 30240395 Free PMC article.
-
[The chemotherapy of Chagas disease].Medicina (B Aires). 1999;59 Suppl 2:147-65. Medicina (B Aires). 1999. PMID: 10668258 Review. Spanish.
Cited by
-
Multiple Cathepsins Promote Pro-IL-1β Synthesis and NLRP3-Mediated IL-1β Activation.J Immunol. 2015 Aug 15;195(4):1685-97. doi: 10.4049/jimmunol.1500509. Epub 2015 Jul 20. J Immunol. 2015. PMID: 26195813 Free PMC article.
-
In vitro and in vivo studies of the trypanocidal properties of WRR-483 against Trypanosoma cruzi.PLoS Negl Trop Dis. 2010 Sep 14;4(9):e825. doi: 10.1371/journal.pntd.0000825. PLoS Negl Trop Dis. 2010. PMID: 20856868 Free PMC article.
-
A nonazole CYP51 inhibitor cures Chagas' disease in a mouse model of acute infection.Antimicrob Agents Chemother. 2010 Jun;54(6):2480-8. doi: 10.1128/AAC.00281-10. Epub 2010 Apr 12. Antimicrob Agents Chemother. 2010. PMID: 20385875 Free PMC article.
-
Identification of Anti-Trypanosoma cruzi Lead Compounds with Putative Immunomodulatory Activity.Antimicrob Agents Chemother. 2018 Mar 27;62(4):e01834-17. doi: 10.1128/AAC.01834-17. Print 2018 Apr. Antimicrob Agents Chemother. 2018. PMID: 29437629 Free PMC article.
-
Proteomics in Trypanosoma cruzi--localization of novel proteins to various organelles.Proteomics. 2008 Jul;8(13):2735-49. doi: 10.1002/pmic.200700940. Proteomics. 2008. PMID: 18546153 Free PMC article.
References
-
- Altcheh, J., M. Biancardi, A. Lapena, G. Ballering, and H. Freilij. 2005. Congenital Chagas disease: experience in the Hospital de Niños, Ricardo Gutierrez, Buenos Aires, Argentina. Rev. Soc. Bras. Med. Trop. 38:41-45. - PubMed
-
- Avila, H., A. M. Goncalves, N. S. Nehme, C. M. Morel, and L. Simpson. 1990. Schizodeme analysis of Trypanosoma cruzi stocks from South and Central America by analysis of PCR-amplified minicircle variable region sequences. Mol. Biochem. Parasitol. 42:175-188. - PubMed
-
- Barcán, L., C. Lunaó, L. Clara, A. Sinagra, A. Valledor, A. M. De Rissio, A. Gadanoá, M. García, E. Santibañes, and A. A. Riarte. 2005. Transmission of T. cruzi infection via liver transplantation to a nonreactive recipient for Chagas' disease. Liver Transplant. 11:1112-1116. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

