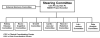
Design and methods of the Chronic Kidney Disease in Children (CKiD) prospective cohort study
- PMID: 17699320
- PMCID: PMC3630231
- DOI: 10.2215/CJN.01941205
Design and methods of the Chronic Kidney Disease in Children (CKiD) prospective cohort study
Abstract
An estimated 650,000 Americans will have ESRD by 2010. Young adults with kidney failure often develop progressive chronic kidney disease (CKD) in childhood and adolescence. The Chronic Kidney Disease in Children (CKiD) prospective cohort study of 540 children aged 1 to 16 yr and have estimated GFR between 30 and 75 ml/min per 1.73 m2 was established to identify novel risk factors for CKD progression; the impact of kidney function decline on growth, cognition, and behavior; and the evolution of cardiovascular disease risk factors. Annually, a physical examination documenting height, weight, Tanner stage, and standardized BP is conducted, and cognitive function, quality of life, nutritional, and behavioral questionnaires are completed by the parent or the child. Samples of serum, plasma, urine, hair, and fingernail clippings are stored in biosamples and genetics repositories. GFR is measured annually for 2 yr, then every other year using iohexol, HPLC creatinine, and cystatin C. Using age, gender, and serial measurements of Tanner stage, height, and creatinine, compared with iohexol GFR, a formula to estimate GFR that will improve on traditional pediatric GFR estimating equations when applied longitudinally is expected to be developed. Every other year, echocardiography and ambulatory BP monitoring will assess risk for cardiovascular disease. The primary outcome is the rate of decline of GFR. The CKiD study will be the largest North American multicenter study of pediatric CKD.
Figures
References
-
- USRDS 2003 Annual Data Report: Atlas of End Stage Renal Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda: 2003.
-
- Feldman HI, Appel LJ, Chertow GM, Cifelli D, Cizman B, Daugirdas J, Fink JC, Franklin-Becker ED, Go AS, Hamm LL, He J, Hostetter T, Hsu CY, Jamerson K, Joffe M, Kusek JW, Landis JR, Lash JP, Miller ER, Mohler ER, III, Muntner P, Ojo AO, Rahman M, Townsend RR, Wright JT. The Chronic Renal Insufficiency Cohort (CRIC) study: Design and methods. J Am Soc Nephrol. 2003;14:S148–S153. Suppl. - PubMed
-
- [Accessed July 6, 2005];Healthy People 2010. 2001 Available online: www.usrds.org/2003/pdf/oc_hp2010_03.pdf.
-
- USRDS 2004 Annual Data Report: Pediatric ESRD. Am J Kidney Dis. 2005;45:S153–S166.
-
- Lin CY, Sheng CC, Chen CH, Lin CC, Chou P. The prevalence of heavy proteinuria and progression risk factors in children undergoing urinary screening. Pediatr Nephrol. 2000;14:953–959. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical