A novel Ca(V)1.2 N terminus expressed in smooth muscle cells of resistance size arteries modifies channel regulation by auxiliary subunits
- PMID: 17699517
- PMCID: PMC2276565
- DOI: 10.1074/jbc.M610623200
A novel Ca(V)1.2 N terminus expressed in smooth muscle cells of resistance size arteries modifies channel regulation by auxiliary subunits
Abstract
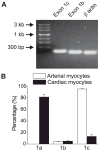
Voltage-dependent L-type Ca(2+) (Ca(V)1.2) channels are the principal Ca(2+) entry pathway in arterial myocytes. Ca(V)1.2 channels regulate multiple vascular functions and are implicated in the pathogenesis of human disease, including hypertension. However, the molecular identity of Ca(V)1.2 channels expressed in myocytes of myogenic arteries that regulate vascular pressure and blood flow is unknown. Here, we cloned Ca(V)1.2 subunits from resistance size cerebral arteries and demonstrate that myocytes contain a novel, cysteine rich N terminus that is derived from exon 1 (termed "exon 1c"), which is located within CACNA1C, the Ca(V)1.2 gene. Quantitative PCR revealed that exon 1c was predominant in arterial myocytes, but rare in cardiac myocytes, where exon 1a prevailed. When co-expressed with alpha(2)delta subunits, Ca(V)1.2 channels containing the novel exon 1c-derived N terminus exhibited: 1) smaller whole cell current density, 2) more negative voltages of half activation (V(1/2,act)) and half-inactivation (V(1/2,inact)), and 3) reduced plasma membrane insertion, when compared with channels containing exon 1b. beta(1b) and beta(2a) subunits caused negative shifts in the V(1/2,act) and V(1/2,inact) of exon 1b-containing Ca(V)1.2alpha(1)/alpha(2)delta currents that were larger than those in exon 1c-containing Ca(V)1.2alpha(1)/alpha(2)delta currents. In contrast, beta(3) similarly shifted V(1/2,act) and V(1/2,inact) of currents generated by exon 1b- and exon 1c-containing channels. beta subunits isoform-dependent differences in current inactivation rates were also detected between N-terminal variants. Data indicate that through novel alternative splicing at exon 1, the Ca(V)1.2 N terminus modifies regulation by auxiliary subunits. The novel exon 1c should generate distinct voltage-dependent Ca(2+) entry in arterial myocytes, resulting in tissue-specific Ca(2+) signaling.
Figures





References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

