Protein kinase CK2 phosphorylation of EB2 regulates its function in the production of Epstein-Barr virus infectious viral particles
- PMID: 17699575
- PMCID: PMC2168784
- DOI: 10.1128/JVI.01421-07
Protein kinase CK2 phosphorylation of EB2 regulates its function in the production of Epstein-Barr virus infectious viral particles
Abstract
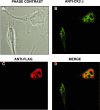
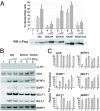
The Epstein-Barr Virus (EBV) early protein EB2 (also called BMLF1, Mta, or SM) promotes the nuclear export of a subset of early and late viral mRNAs and is essential for the production of infectious virions. We show here that in vitro, protein kinase CK2alpha and -beta subunits bind both individually and, more efficiently, as a complex to the EB2 N terminus and that the CK2beta regulatory subunit also interacts with the EB2 C terminus. Immunoprecipitated EB2 has CK2 activity that phosphorylates several sites within the 80 N-terminal amino acids of EB2, including Ser-55, -56, and -57, which are localized next to the nuclear export signal. EB2S3E, the phosphorylation-mimicking mutant of EB2 at these three serines, but not the phosphorylation ablation mutant EB2S3A, efficiently rescued the production of infectious EBV particles by HEK293(BMLF1-KO) cells harboring an EB2-defective EBV genome. The defect of EB2S3A in transcomplementing 293(BMLF1-KO) cells was not due to impaired nucleocytoplasmic shuttling of the mutated protein but was associated with a decrease in the cytoplasmic accumulation of several late viral mRNAs. Thus, EB2-mediated production of infectious EBV virions is regulated by CK2 phosphorylation at one or more of the serine residues Ser-55, -56, and -57.
Figures






References
-
- Aguilera, A. 2005. Cotranscriptional mRNP assembly: from the DNA to the nuclear pore. Curr. Opin. Cell Biol. 17:242-250. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

