Novel adeno-associated virus serotypes efficiently transduce murine photoreceptors
- PMID: 17699581
- PMCID: PMC2045569
- DOI: 10.1128/JVI.01327-07
Novel adeno-associated virus serotypes efficiently transduce murine photoreceptors
Abstract
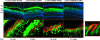
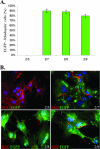
Severe inherited retinal diseases, such as retinitis pigmentosa and Leber congenital amaurosis, are caused by mutations in genes preferentially expressed in photoreceptors. While adeno-associated virus (AAV)-mediated gene transfer can correct retinal pigment epithelium (RPE) defects in animal models, approaches for the correction of photoreceptor-specific diseases are less efficient. We evaluated the ability of novel AAV serotypes (AAV2/7, AAV2/8, AAV2/9, AAV2rh.43, AAV2rh.64R1, and AAV2hu.29R) in combination with constitutive or photoreceptor-specific promoters to improve photoreceptor transduction, a limiting step in photoreceptor rescue. Based on a qualitative analysis, all AAV serotypes tested efficiently transduce the RPE as well as rod and cone photoreceptors after subretinal administration in mice. Interestingly, AAV2/9 efficiently transduces Müller cells. To compare photoreceptor transduction from different AAVs and promoters in both a qualitative and quantitative manner, we designed a strategy based on the use of a bicistronic construct expressing both enhanced green fluorescent protein and luciferase. We found that AAV2/8 and AAV2/7 mediate six- to eightfold higher levels of in vivo photoreceptor transduction than AAV2/5, considered so far the most efficient AAV serotype for photoreceptor targeting. In addition, following subretinal administration of AAV, the rhodopsin promoter allows significantly higher levels of photoreceptor expression than the other ubiquitous or photoreceptor-specific promoters tested. Finally, we show that AAV2/7, AAV2/8, and AAV2/9 outperform AAV2/5 following ex vivo transduction of retinal progenitor cells differentiated into photoreceptors. We conclude that AAV2/7 or AAV2/8 and the rhodopsin promoter provide the highest levels of photoreceptor transduction both in and ex vivo and that this may overcome the limitation to therapeutic success observed so far in models of inherited severe photoreceptor diseases.
Figures



References
-
- Acland, G. M., G. D. Aguirre, J. Bennett, T. S. Aleman, A. V. Cideciyan, J. Bennicelli, N. S. Dejneka, S. E. Pearce-Kelling, A. M. Maguire, K. Palczewski, W. W. Hauswirth, and S. G. Jacobson. 2005. Long-term restoration of rod and cone vision by single dose rAAV-mediated gene transfer to the retina in a canine model of childhood blindness. Mol. Ther. 12:1072-1082. - PMC - PubMed
-
- Acland, G. M., G. D. Aguirre, J. Ray, Q. Zhang, T. S. Aleman, A. V. Cideciyan, S. E. Pearce-Kelling, V. Anand, Y. Zeng, A. M. Maguire, S. G. Jacobson, W. W. Hauswirth, and J. Bennett. 2001. Gene therapy restores vision in a canine model of childhood blindness. Nat. Genet. 28:92-95. - PubMed
-
- Ali, R. R., M. B. Reichel, A. J. Thrasher, R. J. Levinsky, C. Kinnon, N. Kanuga, D. M. Hunt, and S. S. Bhattacharya. 1996. Gene transfer into the mouse retina mediated by an adeno-associated viral vector. Hum. Mol. Genet. 5:591-594. - PubMed
-
- Ali, R. R., G. M. Sarra, C. Stephens, M. D. Alwis, J. W. Bainbridge, P. M. Munro, S. Fauser, M. B. Reichel, C. Kinnon, D. M. Hunt, S. S. Bhattacharya, and A. J. Thrasher. 2000. Restoration of photoreceptor ultrastructure and function in retinal degeneration slow mice by gene therapy. Nat. Genet. 25:306-310. - PubMed
-
- Allocca, M., A. Tessitore, G. Cotugno, and A. Auricchio. 2006. AAV-mediated gene transfer for retinal diseases. Expert Opin. Biol. Ther. 6:1279-1294. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

