Cyclodextrins inhibit replication of scrapie prion protein in cell culture
- PMID: 17699584
- PMCID: PMC2045541
- DOI: 10.1128/JVI.02559-06
Cyclodextrins inhibit replication of scrapie prion protein in cell culture
Abstract
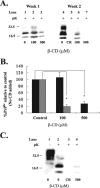
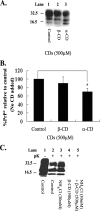
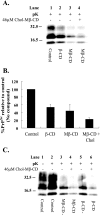
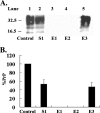
Prion diseases are fatal neurodegenerative disorders that are caused by the conversion of a normal host-encoded protein, PrP(C), to an abnormal, disease-causing form, PrP(Sc). This paper reports that cyclodextrins have the ability to reduce the pathogenic isoform of the prion protein PrP(Sc) to undetectable levels in scrapie-infected neuroblastoma cells. Beta-cyclodextrin removed PrP(Sc) from the cells at a concentration of 500 microM following 2 weeks of treatment. Structure activity studies revealed that antiprion activity was dependent on the size of the cyclodextrin. The half-maximal inhibitory concentration (IC(50)) for beta-cyclodextrin was 75 microM, whereas alpha-cyclodextrin, which possessed less antiprion activity, had an IC(50) of 750 microM. This report presents cyclodextrins as a new class of antiprion compound. For decades, the pharmaceutical industry has successfully used cyclodextrins for their complex-forming ability; this ability is due to the structural orientation of the glucopyranose units, which generate a hydrophobic cavity that can facilitate the encapsulation of hydrophobic moieties. Consequently, cyclodextrins could be ideal candidates for the treatment of prion diseases.
Figures





Similar articles
-
Antiprion action of new cyclodextrin analogues.Biochim Biophys Acta. 2009 Oct;1790(10):1382-6. doi: 10.1016/j.bbagen.2009.07.011. Epub 2009 Jul 23. Biochim Biophys Acta. 2009. PMID: 19631725
-
Charged bipolar suramin derivatives induce aggregation of the prion protein at the cell surface and inhibit PrPSc replication.J Cell Sci. 2005 Nov 1;118(Pt 21):4959-73. doi: 10.1242/jcs.02609. Epub 2005 Oct 11. J Cell Sci. 2005. PMID: 16219680
-
Acridine and phenothiazine derivatives as pharmacotherapeutics for prion disease.Proc Natl Acad Sci U S A. 2001 Aug 14;98(17):9836-41. doi: 10.1073/pnas.161274798. Proc Natl Acad Sci U S A. 2001. PMID: 11504948 Free PMC article.
-
[Mechanisms of prion transmission].Nihon Rinsho. 2007 Aug;65(8):1391-5. Nihon Rinsho. 2007. PMID: 17695274 Review. Japanese.
-
Searching for anti-prion compounds: cell-based high-throughput in vitro assays and animal testing strategies.Methods Enzymol. 2006;412:223-34. doi: 10.1016/S0076-6879(06)12014-5. Methods Enzymol. 2006. PMID: 17046661 Review.
Cited by
-
Efficacy and mechanism of a glycoside compound inhibiting abnormal prion protein formation in prion-infected cells: implications of interferon and phosphodiesterase 4D-interacting protein.J Virol. 2014 Apr;88(8):4083-99. doi: 10.1128/JVI.03775-13. Epub 2014 Jan 22. J Virol. 2014. PMID: 24453367 Free PMC article.
-
Exploring immunotherapeutic strategies for neurodegenerative diseases: a focus on Huntington's disease and Prion diseases.Acta Pharmacol Sin. 2025 Jun;46(6):1511-1538. doi: 10.1038/s41401-024-01455-w. Epub 2025 Jan 31. Acta Pharmacol Sin. 2025. PMID: 39890942 Review.
-
A cell-biased effect of estrogen in prion infection.J Virol. 2014 Jan;88(2):1342-53. doi: 10.1128/JVI.02936-13. Epub 2013 Nov 13. J Virol. 2014. PMID: 24227845 Free PMC article.
-
Human cerebral organoids as a therapeutic drug screening model for Creutzfeldt-Jakob disease.Sci Rep. 2021 Mar 9;11(1):5165. doi: 10.1038/s41598-021-84689-6. Sci Rep. 2021. PMID: 33727594 Free PMC article.
-
Inhibition of cholesterol recycling impairs cellular PrP(Sc) propagation.Cell Mol Life Sci. 2009 Dec;66(24):3979-91. doi: 10.1007/s00018-009-0158-4. Epub 2009 Oct 13. Cell Mol Life Sci. 2009. PMID: 19823766 Free PMC article.
References
-
- Abulrob, A., J. S. Tauskela, G. Mealing, E. Brunette, K. Faid, and D. Stanimirovic. 2005. Protection by cholesterol-extracting cyclodextrins: a role for N-methyl-D-aspartate receptor redistribution. J. Neurochem. 92:1477-1486. - PubMed
-
- Adjou, K. T., R. Demaimay, J. P. Deslys, C. I. Lasmezas, V. Beringue, S. Demart, F. Lamoury, M. Seman, and D. Dormont. 1999. MS-8209, a water-soluble amphotericin B derivative, affects both scrapie agent replication and PrPres accumulation in Syrian hamster scrapie. J. Gen. Virol. 80:1079-1085. - PubMed
-
- Arispe, N., and M. Doh. 2002. Plasma membrane cholesterol controls the cytotoxicity of Alzheimer's disease AbetaP (1-40) and (1-42) peptides. FASEB J. 16:1526-1536. - PubMed
-
- Barenholz, Y. 2004. Sphingomyelin and cholesterol: from membrane biophysics and rafts to potential medical applications. Subcell. Biochem. 37:167-215. - PubMed
-
- Bate, C., M. Salmona, L. Diomede, and A. Williams. 2004. Squalestatin cures prion-infected neurons and protects against prion neurotoxicity. J. Biol. Chem. 279:14983-14990. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

