Multiple myosins are required to coordinate actin assembly with coat compression during compensatory endocytosis
- PMID: 17699600
- PMCID: PMC1995739
- DOI: 10.1091/mbc.e06-11-0993
Multiple myosins are required to coordinate actin assembly with coat compression during compensatory endocytosis
Abstract
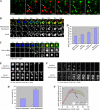
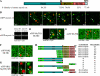
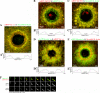
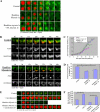
Actin is involved in endocytosis in organisms ranging from yeast to mammals. In activated Xenopus eggs, exocytosing cortical granules (CGs) are surrounded by actin "coats," which compress the exocytosing compartments, resulting in compensatory endocytosis. Here, we examined the roles of two myosins in actin coat compression. Myosin-2 is recruited to exocytosing CGs late in coat compression. Inhibition of myosin-2 slows coat compression without affecting actin assembly. This differs from phenotype induced by inhibition of actin assembly, where exocytosing CGs are trapped at the plasma membrane (PM) completely. Thus, coat compression is likely driven in part by actin assembly itself, but it requires myosin-2 for efficient completion. In contrast to myosin-2, the long-tailed myosin-1e is recruited to exocytosing CGs immediately after egg activation. Perturbation of myosin-1e results in partial actin coat assembly and induces CG collapse into the PM. Intriguingly, simultaneous inhibition of actin assembly and myosin-1e prevents CG collapse. Together, the results show that myosin-1e and myosin-2 are part of an intricate machinery that coordinates coat compression at exocytosing CGs.
Figures


Similar articles
-
Myosin-1c couples assembling actin to membranes to drive compensatory endocytosis.Dev Cell. 2006 Nov;11(5):629-40. doi: 10.1016/j.devcel.2006.09.002. Dev Cell. 2006. PMID: 17084356 Free PMC article.
-
Control of local actin assembly by membrane fusion-dependent compartment mixing.Nat Cell Biol. 2007 Feb;9(2):149-59. doi: 10.1038/ncb1527. Epub 2007 Jan 21. Nat Cell Biol. 2007. PMID: 17237773 Free PMC article.
-
Cdc42-dependent actin polymerization during compensatory endocytosis in Xenopus eggs.Nat Cell Biol. 2003 Aug;5(8):727-32. doi: 10.1038/ncb1025. Nat Cell Biol. 2003. PMID: 12872130
-
Kiss-and-coat and compartment mixing: coupling exocytosis to signal generation and local actin assembly.Mol Biol Cell. 2006 Apr;17(4):1495-502. doi: 10.1091/mbc.e05-10-0908. Epub 2006 Jan 25. Mol Biol Cell. 2006. PMID: 16436510 Free PMC article. Review.
-
Membrane shaping by actin and myosin during regulated exocytosis.Mol Cell Neurosci. 2017 Oct;84:93-99. doi: 10.1016/j.mcn.2017.05.006. Epub 2017 May 20. Mol Cell Neurosci. 2017. PMID: 28536001 Review.
Cited by
-
Compensatory endocytosis in bladder umbrella cells occurs through an integrin-regulated and RhoA- and dynamin-dependent pathway.EMBO J. 2010 Jun 16;29(12):1961-75. doi: 10.1038/emboj.2010.91. Epub 2010 May 11. EMBO J. 2010. PMID: 20461056 Free PMC article.
-
Real-time measurement of F-actin remodelling during exocytosis using Lifeact-EGFP transgenic animals.PLoS One. 2012;7(7):e39815. doi: 10.1371/journal.pone.0039815. Epub 2012 Jul 2. PLoS One. 2012. PMID: 22768313 Free PMC article.
-
Long-Tailed Unconventional Class I Myosins in Health and Disease.Int J Mol Sci. 2020 Apr 7;21(7):2555. doi: 10.3390/ijms21072555. Int J Mol Sci. 2020. PMID: 32272642 Free PMC article. Review.
-
Actomyosin II contractility expels von Willebrand factor from Weibel-Palade bodies during exocytosis.J Cell Biol. 2011 Aug 22;194(4):613-29. doi: 10.1083/jcb.201011119. Epub 2011 Aug 15. J Cell Biol. 2011. PMID: 21844207 Free PMC article.
-
Myosin-1C augments endothelial secretion of von Willebrand factor by linking contractile actomyosin machinery to the plasma membrane.Blood Adv. 2024 Sep 10;8(17):4714-4726. doi: 10.1182/bloodadvances.2024012590. Blood Adv. 2024. PMID: 38669344 Free PMC article.
References
-
- Becker K. A., Hart N. H. Reorganization of filamentous actin and myosin-II in zebrafish eggs correlates temporally and spatially with cortical granule exocytosis. J. Cell Sci. 1999;112:97–110. - PubMed
-
- Bose A., Guilherme A., Robida S. I., Nicoloro S. M., Zhou Q. L., Jiang Z. Y., Pomerleau D. P., Czech M. P. Glucose transporter recycling in response to insulin is facilitated by myosin Myo1c. Nature. 2002;420:821–824. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

