Excess vacuolar SNAREs drive lysis and Rab bypass fusion
- PMID: 17699614
- PMCID: PMC1959418
- DOI: 10.1073/pnas.0704741104
Excess vacuolar SNAREs drive lysis and Rab bypass fusion
Abstract
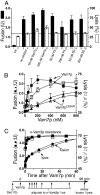
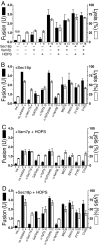
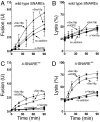
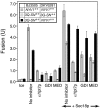
Although concentrated soluble N-ethylmaleimide-sensitive factor attachment protein receptors (SNAREs) drive liposome fusion and lysis, the fusion of intracellular membranes also requires Rab GTPases, Rab effectors, SM proteins, and specific regulatory lipids and is accompanied by little or no lysis. To rationalize these findings, we generated yeast strains that overexpress all four vacuolar SNAREs (4SNARE(++)). Although vacuoles with physiological levels of Rab, Rab effector/SM complex, and SNAREs support rapid fusion without Rab- and SNARE-dependent lysis, vacuoles from 4SNARE(++) strains show extensive lysis and a reduced need for the Rab Ypt7p or regulatory lipids for fusion. SNARE overexpression and the addition of pure homotypic fusion and vacuole protein sorting complex (HOPS), which bears the vacuolar SM protein, enables ypt7Delta vacuoles to fuse, allowing direct comparison of Rab-dependent and Rab-independent fusion. Because 3- to 40-fold more of each of the five components that form the SNARE/HOPS fusion complex are required for vacuoles from ypt7Delta strains to fuse at the same rate as vacuoles from wild-type strains, the apparent forward rate constant of 4SNARE/HOPS complex assembly is enhanced many thousand-fold by Ypt7p. Rabs function in normal membrane fusion by concentrating SNAREs, other proteins (e.g., SM), and key lipids at a fusion site and activating them for fusion without lysis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Comment in
-
Membrane fusion as a team effort.Proc Natl Acad Sci U S A. 2007 Aug 21;104(34):13541-2. doi: 10.1073/pnas.0706168104. Epub 2007 Aug 15. Proc Natl Acad Sci U S A. 2007. PMID: 17699625 Free PMC article. No abstract available.
Similar articles
-
The major role of the Rab Ypt7p in vacuole fusion is supporting HOPS membrane association.J Biol Chem. 2009 Jun 12;284(24):16118-16125. doi: 10.1074/jbc.M109.000737. Epub 2009 Apr 21. J Biol Chem. 2009. PMID: 19386605 Free PMC article.
-
Minimal membrane docking requirements revealed by reconstitution of Rab GTPase-dependent membrane fusion from purified components.Proc Natl Acad Sci U S A. 2009 Oct 20;106(42):17626-33. doi: 10.1073/pnas.0903801106. Epub 2009 Oct 13. Proc Natl Acad Sci U S A. 2009. PMID: 19826089 Free PMC article.
-
Membrane fusion catalyzed by a Rab, SNAREs, and SNARE chaperones is accompanied by enhanced permeability to small molecules and by lysis.Mol Biol Cell. 2011 Dec;22(23):4635-46. doi: 10.1091/mbc.E11-08-0680. Epub 2011 Oct 5. Mol Biol Cell. 2011. PMID: 21976702 Free PMC article.
-
Membrane fusion: five lipids, four SNAREs, three chaperones, two nucleotides, and a Rab, all dancing in a ring on yeast vacuoles.Annu Rev Cell Dev Biol. 2010;26:115-36. doi: 10.1146/annurev-cellbio-100109-104131. Annu Rev Cell Dev Biol. 2010. PMID: 20521906 Review.
-
Membrane dynamics and fusion at late endosomes and vacuoles--Rab regulation, multisubunit tethering complexes and SNAREs.Eur J Cell Biol. 2011 Sep;90(9):779-85. doi: 10.1016/j.ejcb.2011.04.007. Epub 2011 Jun 16. Eur J Cell Biol. 2011. PMID: 21683469 Review.
Cited by
-
Yeast lunapark regulates the formation of trans-Sey1p complexes for homotypic ER membrane fusion.iScience. 2023 Nov 2;26(12):108386. doi: 10.1016/j.isci.2023.108386. eCollection 2023 Dec 15. iScience. 2023. PMID: 38025788 Free PMC article.
-
A LAPF/phafin1-like protein regulates TORC1 and lysosomal membrane permeabilization in response to endoplasmic reticulum membrane stress.Mol Biol Cell. 2015 Dec 15;26(25):4631-45. doi: 10.1091/mbc.E15-08-0581. Epub 2015 Oct 28. Mol Biol Cell. 2015. PMID: 26510498 Free PMC article.
-
Yeast lipin 1 orthologue pah1p regulates vacuole homeostasis and membrane fusion.J Biol Chem. 2012 Jan 13;287(3):2221-36. doi: 10.1074/jbc.M111.317420. Epub 2011 Nov 25. J Biol Chem. 2012. PMID: 22121197 Free PMC article.
-
A lipid-anchored SNARE supports membrane fusion.Proc Natl Acad Sci U S A. 2011 Oct 18;108(42):17325-30. doi: 10.1073/pnas.1113888108. Epub 2011 Oct 10. Proc Natl Acad Sci U S A. 2011. PMID: 21987819 Free PMC article.
-
An abscisic acid-induced protein, HVA22, inhibits gibberellin-mediated programmed cell death in cereal aleurone cells.Plant Physiol. 2008 Aug;147(4):1710-22. doi: 10.1104/pp.108.120238. Epub 2008 Jun 26. Plant Physiol. 2008. PMID: 18583533 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

