Dissociation of the insulin receptor and caveolin-1 complex by ganglioside GM3 in the state of insulin resistance
- PMID: 17699617
- PMCID: PMC1949342
- DOI: 10.1073/pnas.0703650104
Dissociation of the insulin receptor and caveolin-1 complex by ganglioside GM3 in the state of insulin resistance
Abstract
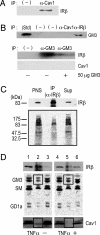
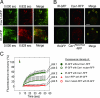
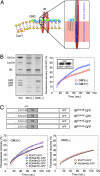
Membrane microdomains (lipid rafts) are now recognized as critical for proper compartmentalization of insulin signaling. We previously demonstrated that, in adipocytes in a state of TNFalpha-induced insulin resistance, the inhibition of insulin metabolic signaling and the elimination of insulin receptors (IR) from the caveolae microdomains were associated with an accumulation of the ganglioside GM3. To gain insight into molecular mechanisms behind interactions of IR, caveolin-1 (Cav1), and GM3 in adipocytes, we have performed immunoprecipitations, cross-linking studies of IR and GM3, and live cell studies using total internal reflection fluorescence microscopy and fluorescence recovery after photobleaching techniques. We found that (i) IR form complexes with Cav1 and GM3 independently; (ii) in GM3-enriched membranes the mobility of IR is increased by dissociation of the IR-Cav1 interaction; and (iii) the lysine residue localized just above the transmembrane domain of the IR beta-subunit is essential for the interaction of IR with GM3. Because insulin metabolic signal transduction in adipocytes is known to be critically dependent on caveolae, we propose a pathological feature of insulin resistance in adipocytes caused by dissociation of the IR-Cav1 complex by the interactions of IR with GM3 in microdomains.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
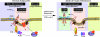
References
-
- Nojiri H, Stroud M, Hakomori S. J Biol Chem. 1991;266:4531–4537. - PubMed
-
- Tagami S, Inokuchi J-i, Kabayama K, Yoshimura H, Kitamura F, Uemura S, Ogawa C, Ishii A, Saito M, Ohtsuka Y, et al. J Biol Chem. 2002;277:3085–3092. - PubMed
-
- Razani B, Woodman SE, Lisanti MP. Pharmacol Rev. 2002;54:431–467. - PubMed
-
- Goldberg RI, Smith RM, Jarett L. J Cell Physiol. 1987;133:203–212. - PubMed
-
- Gustavsson J, Parpal S, Karlsson M, Ramsing C, Thorn H, Borg M, Lindroth M, Peterson KH, Magnusson KE, Stralfors P. FASEB J. 1999;13:1961–1971. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

