An inhibitor of viral RNA replication is encoded by a plant resistance gene
- PMID: 17699618
- PMCID: PMC1949341
- DOI: 10.1073/pnas.0703203104
An inhibitor of viral RNA replication is encoded by a plant resistance gene
Abstract
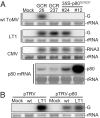
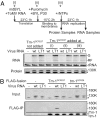
The tomato Tm-1 gene confers resistance to tomato mosaic virus (ToMV). Here, we report that the extracts of Tm-1 tomato cells (GCR237) have properties that inhibit the in vitro RNA replication of WT ToMV more strongly than that of the Tm-1-resistance-breaking mutant of ToMV, LT1. We purified this inhibitory activity and identified a polypeptide of approximately 80 kDa (p80(GCR237)) using LC-tandem MS. The amino acid sequence of p80(GCR237) had no similarity to any characterized proteins. The p80(GCR237) gene cosegregated with Tm-1; transgenic expression of p80(GCR237) conferred resistance to ToMV within tomato plants; and the knockdown of p80(GCR237) sensitized Tm-1 tomato plants to ToMV, indicating that Tm-1 encodes p80(GCR237) itself. We further show that in vitro-synthesized Tm-1 (p80(GCR237)) protein binds to the replication proteins of WT ToMV and inhibits their function at a step before, but not after, the viral replication complex is formed on the membrane surfaces. Such binding was not observed for the replication proteins of LT1. These results suggest that Tm-1 (p80(GCR237)) inhibits the replication of WT ToMV RNA through binding to the replication proteins.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Kang BC, Yeam I, Jahn MM. Annu Rev Phytopathol. 2005;43:581–621. - PubMed
-
- Diaz-Pendon JA, Truniger V, Nieto C, Garcia-Mas J, Bendahmane A, Aranda MA. Mol Plant Pathol. 2004;5:223–233. - PubMed
-
- Motoyoshi F, Oshima N. J Gen Virol. 1977;34:499–506. - PubMed
-
- Fraser RSS, Loughlin SAR. J Gen Virol. 1980;48:87–96.
-
- Pelham J. Euphytica. 1966;15:258–267.
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

