Strategic approach to produce low-cost, efficient, and stable competitive internal controls for detection of RNA viruses by use of reverse transcription-PCR
- PMID: 17699653
- PMCID: PMC2168486
- DOI: 10.1128/JCM.02601-06
Strategic approach to produce low-cost, efficient, and stable competitive internal controls for detection of RNA viruses by use of reverse transcription-PCR
Abstract
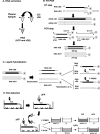
Molecular diagnostics based on reverse transcription (RT)-PCR are routinely complicated by the lack of stable internal controls, leading to falsely negative results. We describe a strategy to produce a stable competitive internal control (CIC) based on a Qbeta phage derivative (recombinant Qbeta [rQbeta]) bearing primers KY78 and KY80, which are widely used in the detection of hepatitis C virus (HCV). rQbeta was RNase resistant and stable at 4 degrees C for 452 days in SM medium (0.1 M NaCl, 8 mM MgSO(4).7H(2)O, 50 mM Tris HCl [pH 7.5], 2% gelatin) and for 125 days after lyophilization and reconstitution. rQbeta performance as a CIC was evaluated. rQbeta was added to HCV-positive samples, followed by RNA extraction and a CIC-HCV RT-PCR assay. This method combines RT-PCR, liquid hybridization with nonradioactive probes, and enzyme immunoanalysis. No influence of the CIC on qualitative HCV detection was observed independently of viral load, and results had high concordance with those of commercial kits. In conclusion, we describe a versatile, low-cost alternative strategy to armored RNA technology that can be adapted for detection or real-time applications of any RNA target. Moreover, the CIC reported here is an essential reagent for HCV screening in blood banks in resource-limited settings.
Figures

References
-
- Asociación Argentina para el Estudio de las Enfermedades del Hígado. 2004. Consenso Argentino hepatitis C. Asociación Argentina para el Estudio de las Enfermedades del Hígado, Buenos Aires, Argentina. http://www.aaeeh.org.ar/conce2004.php.
-
- Beld, M., R. Minnaar, J. Weel, C. Sol, M. Damen, H. van der Avoort, P. Wertheim-van Dillen, A. van Breda, and R. Boom. 2004. Highly sensitive assay for detection of enterovirus in clinical specimens by reverse transcription-PCR with an armored RNA internal control. J. Clin. Microbiol. 42:3059-3064. - PMC - PubMed
-
- Blackwell Publishing Ltd. 1999. Global surveillance and control of hepatitis C. Report of a WHO consultation organized in collaboration with the Viral Hepatitis Prevention Board, Antwerp, Belgium. J. Viral Hepat. 6:35-47. - PubMed
-
- Chouhy, D., L. Benitez Gil, A. L. Nocito, D. Wojdyla, L. Ornella, J. Cittadini, D. Gardiol, and A. A. Giri. 2006. Development and evaluation of a colorimetric PCR system for the detection and typing of human papillomaviruses. Int. J. Mol. Med. 18:995-1003. - PubMed
-
- Cleland, A., P. Nettleton, L. Jarvis, and P. Simmonds. 1999. Use of bovine viral diarrhoea virus as an internal control for amplification of hepatitis C virus. Vox Sang. 76:170-174. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

