Constitutive excitation by Gly90Asp rhodopsin rescues rods from degeneration caused by elevated production of cGMP in the dark
- PMID: 17699662
- PMCID: PMC2673730
- DOI: 10.1523/JNEUROSCI.2751-07.2007
Constitutive excitation by Gly90Asp rhodopsin rescues rods from degeneration caused by elevated production of cGMP in the dark
Abstract
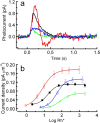
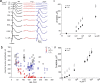
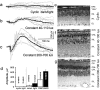
Previous experiments indicate that congenital human retinal degeneration caused by genetic mutations that change the Ca(2+) sensitivity of retinal guanylyl cyclase (retGC) can result from an increase in concentration of free intracellular cGMP and Ca(2+) in the photoreceptors. To rescue degeneration in transgenic mouse models having either the Y99C or E155G mutations of the retGC modulator guanylyl cyclase-activating protein 1 (GCAP-1), which produce elevated cGMP synthesis in the dark, we used the G90D rhodopsin mutation, which produces constitutive stimulation of cGMP hydrolysis. The effects of the G90D transgene were evaluated by measuring retGC activity biochemically, by recording single rod and electroretinogram (ERG) responses, by intracellular free Ca(2+) measurement, and by retinal morphological analysis. Although the G90D rhodopsin did not alter the abnormal Ca(2+) sensitivity of retGC in the double-mutant animals, the intracellular free cGMP and Ca(2+) concentrations returned close to normal levels, consistent with constitutive activation of the phosphodiesterase PDE6 cascade in darkness. G90D decreased the light sensitivity of rods but spared them from severe retinal degeneration in Y99C and E155G GCAP-1 mice. More than half of the photoreceptors remained alive, appeared morphologically normal, and produced electrical responses, at the time when their siblings lacking the G90D rhodopsin transgene lost the entire retinal outer nuclear layer and no longer responded to illumination. These experiments indicate that mutations that lead to increases in cGMP and Ca(2+) can trigger photoreceptor degeneration but that constitutive activation of the transduction cascade in these animals can greatly enhance cell survival.
Figures





References
-
- Brooks SP, Storey KB. Bound and determined: a computer program for making buffers of defined ion concentrations. Anal Biochem. 1992;201:119–126. - PubMed
-
- Burns ME, Arshavsky VY. Beyond counting photons: trials and trends in vertebrate visual transduction. Neuron. 2005;48:387–401. - PubMed
-
- Burns ME, Mendez A, Chen J, Baylor DA. Dynamics of cyclic GMP synthesis in retinal rods. Neuron. 2002;36:81–91. - PubMed
-
- Calvert PD, Govardovskii VI, Krasnoperova N, Anderson RE, Lem J, Makino CL. Membrane protein diffusion sets the speed of rod phototransduction. Nature. 2001;411:90–94. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
