Long-term consequences of methamphetamine exposure in young adults are exacerbated in glial cell line-derived neurotrophic factor heterozygous mice
- PMID: 17699663
- PMCID: PMC2698457
- DOI: 10.1523/JNEUROSCI.1067-07.2007
Long-term consequences of methamphetamine exposure in young adults are exacerbated in glial cell line-derived neurotrophic factor heterozygous mice
Abstract
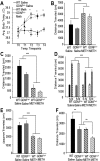
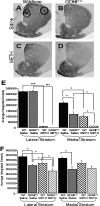
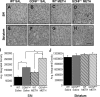
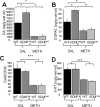
Methamphetamine abuse in young adults has long-term deleterious effects on brain function that are associated with damage to monoaminergic neurons. Administration of glial cell line-derived neurotrophic factor (GDNF) protects dopamine neurons from the toxic effects of methamphetamine in animal models. Therefore, we hypothesized that a partial GDNF gene deletion would increase the susceptibility of mice to methamphetamine neurotoxicity during young adulthood and possibly increase age-related deterioration of behavior and dopamine function. Two weeks after a methamphetamine binge (4 x 10 mg/kg, i.p., at 2 h intervals), GDNF(+/-) mice had a significantly greater reduction of tyrosine hydroxylase immunoreactivity in the medial striatum, a proportionally greater depletion of dopamine and 3,4-dihydroxyphenylacetic acid (DOPAC) levels in the striatum, and a greater increase in activated microglia in the substantia nigra than wild-type mice. At 12 months of age, methamphetamine-treated GDNF(+/-) mice exhibited less motor activity and lower levels of tyrosine hydroxylase-immunoreactivity, dopamine, DOPAC, and serotonin than wild-type mice. Greater striatal dopamine transporter activity in GDNF(+/-) mice may underlie their differential response to methamphetamine. These data suggest the possibility that methamphetamine use in young adults, when combined with lower levels of GDNF throughout life, may precipitate the appearance of parkinsonian-like behaviors during aging.
Figures


Similar articles
-
Minocycline restores striatal tyrosine hydroxylase in GDNF heterozygous mice but not in methamphetamine-treated mice.Neurobiol Dis. 2009 Mar;33(3):459-66. doi: 10.1016/j.nbd.2008.11.013. Epub 2008 Dec 9. Neurobiol Dis. 2009. PMID: 19110059 Free PMC article.
-
Telmisartan attenuates MPTP induced dopaminergic degeneration and motor dysfunction through regulation of α-synuclein and neurotrophic factors (BDNF and GDNF) expression in C57BL/6J mice.Neuropharmacology. 2013 Oct;73:98-110. doi: 10.1016/j.neuropharm.2013.05.025. Epub 2013 Jun 6. Neuropharmacology. 2013. PMID: 23747572
-
Glial cell-line derived neurotrophic factor (GDNF) replacement attenuates motor impairments and nigrostriatal dopamine deficits in 12-month-old mice with a partial deletion of GDNF.Pharmacol Biochem Behav. 2013 Mar;104:10-9. doi: 10.1016/j.pbb.2012.12.022. Epub 2013 Jan 2. Pharmacol Biochem Behav. 2013. PMID: 23290934 Free PMC article.
-
Impact of methamphetamine on dopamine neurons in primates is dependent on age: implications for development of Parkinson's disease.Neuroscience. 2011 Aug 25;189:277-85. doi: 10.1016/j.neuroscience.2011.05.046. Epub 2011 Jun 1. Neuroscience. 2011. PMID: 21640165 Free PMC article.
-
Glial cell line-derived neurotrophic factor is crucial for long-term maintenance of the nigrostriatal system.Neuroscience. 2010 Dec 29;171(4):1357-66. doi: 10.1016/j.neuroscience.2010.10.010. Epub 2010 Oct 8. Neuroscience. 2010. PMID: 20933580 Free PMC article.
Cited by
-
Nucleus accumbens invulnerability to methamphetamine neurotoxicity.ILAR J. 2011;52(3):352-65. doi: 10.1093/ilar.52.3.352. ILAR J. 2011. PMID: 23382149 Free PMC article. Review.
-
Effects of Neurotrophic Factors in Glial Cells in the Central Nervous System: Expression and Properties in Neurodegeneration and Injury.Front Physiol. 2019 Apr 26;10:486. doi: 10.3389/fphys.2019.00486. eCollection 2019. Front Physiol. 2019. PMID: 31105589 Free PMC article. Review.
-
5-HT1F receptor-mediated mitochondrial biogenesis for the treatment of Parkinson's disease.Br J Pharmacol. 2018 Jan;175(2):348-358. doi: 10.1111/bph.14076. Epub 2017 Dec 22. Br J Pharmacol. 2018. PMID: 29057453 Free PMC article.
-
The glial cell modulator and phosphodiesterase inhibitor, AV411 (ibudilast), attenuates prime- and stress-induced methamphetamine relapse.Eur J Pharmacol. 2010 Jul 10;637(1-3):102-8. doi: 10.1016/j.ejphar.2010.04.010. Epub 2010 Apr 23. Eur J Pharmacol. 2010. PMID: 20399770 Free PMC article.
-
Short-term effects of an endotoxin on substantia nigra dopamine neurons.Brain Res. 2014 Apr 4;1557:164-70. doi: 10.1016/j.brainres.2014.02.005. Epub 2014 Feb 8. Brain Res. 2014. PMID: 24513404 Free PMC article.
References
-
- Bakhit C, Morgan ME, Peat MA, Gibb JW. Long-term effects of methamphetamine on the synthesis and metabolism of 5-hydroxytryptamine in various regions of the rat brain. Neuropharmacology. 1981;20:1135–1140. - PubMed
-
- Boger HA, Granholm AC, Jin L, Nelson ME, Page GP, McGinty JF. Striatal gene expression profile of 12-month-old GDNF heterozygous mice. Soc Neurosci Abstr. 2004;30:725–17.
-
- Boger HA, Middaugh LD, Huang P, Zaman V, Smith AC, Hoffer BJ, Tomac AC, Granholm AC. A partial GDNF depletion leads to earlier age-related deterioration of motor function and tyrosine hydroxylase expression in the substantia nigra. Exp Neurol. 2006;202:336–347. - PubMed
-
- Bowenkamp KE, Hoffman AF, Gerhardt GA, Henry MA, Biddle PT, Hoffer BJ, Granholm AC. Glial cell line-derived neurotrophic factor supports survival of injured midbrain dopaminergic neurons. J Comp Neurol. 1995;355:479–489. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
