The kisspeptin receptor GPR54 is required for sexual differentiation of the brain and behavior
- PMID: 17699664
- PMCID: PMC6672184
- DOI: 10.1523/JNEUROSCI.2099-07.2007
The kisspeptin receptor GPR54 is required for sexual differentiation of the brain and behavior
Abstract
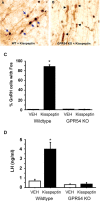
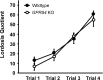
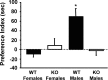
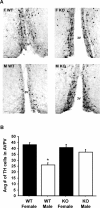
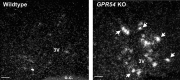
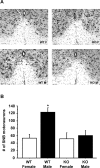
GPR54 is a G-protein-coupled receptor, which binds kisspeptins and is widely expressed throughout the brain. Kisspeptin-GPR54 signaling has been implicated in the regulation of pubertal and adulthood gonadotropin-releasing hormone (GnRH) secretion, and mutations or deletions of GPR54 cause hypogonadotropic hypogonadism in humans and mice. Other reproductive roles for kisspeptin-GPR54 signaling, including the regulation of developmental GnRH secretion or sexual behavior in adults, have not yet been explored. Using adult wild-type (WT) and GPR54 knock-out (KO) mice, we first tested whether kisspeptin-GPR54 signaling is necessary for male and female sexual behaviors. We found that hormone-replaced gonadectomized GPR54 KO males and females displayed appropriate gender-specific adult sexual behaviors. Next, we examined whether GPR54 signaling is required for proper display of olfactory-mediated partner preference behavior. Testosterone-treated WT males preferred stimulus females rather than males, whereas similarly treated WT females and GPR54 KO males showed no preference for either sex. Because olfactory preference is sexually dimorphic and organized during development by androgens, we assessed whether GPR54 signaling is essential for sexual differentiation of other sexually dimorphic traits. Interestingly, adult testosterone-treated GPR54 KO males displayed "female-like" numbers of tyrosine hydroxylase-immunoreactive and Kiss1 mRNA-containing neurons in the anteroventral periventricular nucleus and likewise possessed fewer motoneurons in the spino-bulbocavernosus nucleus than did WT males. Our findings indicate that kisspeptin-GPR54 signaling is not required for male or female copulatory behavior, provided there is appropriate adulthood hormone replacement. However, GPR54 is necessary for proper male-like development of several sexually dimorphic traits, likely by regulating GnRH-mediated androgen secretion during "critical windows" in perinatal development.
Figures
References
-
- Bakker J, Honda S, Harada N, Balthazart J. Restoration of male sexual behavior by adult exogenous estrogens in male aromatase knockout mice. Horm Behav. 2004;46:1–10. - PubMed
-
- Bakker J, De Mees C, Szpirer J, Szpirer C, Balthazart J. Exposure to oestrogen prenatally does not interfere with the normal female-typical development of odour preferences. J Neuroendocrinol. 2007;19:329–334. - PubMed
-
- Baum MJ. Differentiation of coital behavior in mammals: a comparative analysis. Neurosci Biobehav Rev. 1979;3:265–284. - PubMed
-
- Baum MJ, Keverne EB. Sex difference in attraction thresholds for volatile odors from male and estrous female mouse urine. Horm Behav. 2002;41:213–219. - PubMed
-
- Bodo C, Rissman EF. Androgen receptor is essential for sexual differentiation of responses to olfactory cues in mice. Eur J Neurosci. 2007;25:2182–2190. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 DK007646/DK/NIDDK NIH HHS/United States
- T32 HD07382/HD/NICHD NIH HHS/United States
- R01 HD27142/HD/NICHD NIH HHS/United States
- R01 MH057759/MH/NIMH NIH HHS/United States
- U54 HD28934/HD/NICHD NIH HHS/United States
- U54 HD028934/HD/NICHD NIH HHS/United States
- U54 HD012629/HD/NICHD NIH HHS/United States
- T32 HD007382/HD/NICHD NIH HHS/United States
- F32 MH070084/MH/NIMH NIH HHS/United States
- U54 HD12629/HD/NICHD NIH HHS/United States
- T32 DK07646/DK/NIDDK NIH HHS/United States
- R01 MH57759/MH/NIMH NIH HHS/United States
- R01 HD027142/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
