TrpC3/C7 and Slo2.1 are molecular targets for metabotropic glutamate receptor signaling in rat striatal cholinergic interneurons
- PMID: 17699666
- PMCID: PMC6672182
- DOI: 10.1523/JNEUROSCI.0551-07.2007
TrpC3/C7 and Slo2.1 are molecular targets for metabotropic glutamate receptor signaling in rat striatal cholinergic interneurons
Abstract
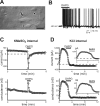
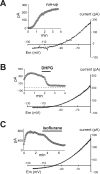
Large aspiny cholinergic interneurons provide the sole source of striatal acetylcholine, a neurotransmitter critical for basal ganglia function; these tonically active interneurons receive excitatory inputs from corticostriatal glutamatergic afferents that act, in part, via metabotropic glutamate receptors (mGluRs). We combined electrophysiological recordings in brain slices with molecular neuroanatomy to identify distinct ion channel targets for mGluR1/5 receptors in striatal cholinergic interneurons: transient receptor potential channel 3/7 (TrpC3/C7) and Slo2.1. In recordings obtained with methanesulfonate-based internal solutions, we found an mGluR-activated current with voltage-dependent and pharmacological properties reminiscent of TrpC3 and TrpC7; expression of these TrpC subunits in cholinergic interneurons was verified by combined immunohistochemistry and in situ hybridization, and modulation of both TrpC channels was reconstituted in HEK293 (human embryonic kidney 293) cells cotransfected with mGluR1 or mGluR5. With a chloride-based internal solution, mGluR agonists did not activate interneuron TrpC-like currents. Instead, a time-dependent, outwardly rectifying K(+) current developed after whole-cell access, and this Cl(-)-activated K(+) current was strongly inhibited by volatile anesthetics and mGluR activation. This modulation was recapitulated in cells transfected with Slo2.1, a Na(+)- and Cl(-)-activated K(+) channel, and Slo2.1 expression was confirmed histochemically in striatal cholinergic interneurons. By using gramicidin perforated-patch recordings, we established that the predominant agonist-activated current was TrpC-like when ambient intracellular chloride was preserved, although a small K(+) current contribution was observed in some cells. Together, our data indicate that mGluR1/5-mediated glutamatergic excitation of cholinergic interneurons is primarily a result of activation of TrpC3/TrpC7-like cationic channels; under conditions when intracellular NaCl is elevated, a Slo2.1 background K(+) channel may also contribute.
Figures







References
-
- Annoura H, Fukunaga A, Uesugi M, Tatsuoka T, Horikawa Y. A novel class of antagonists for metabotropic glutamate receptors, 7-(hydroxyimino)cyclopropa[b]chromen-1a-carboxylates. Bioorg Med Chem Lett. 1996;6:763–766.
-
- Bell MI, Richardson PJ, Lee K. Functional and molecular characterization of metabotropic glutamate receptors expressed in rat striatal cholinergic interneurones. J Neurochem. 2002;81:142–149. - PubMed
-
- Berg AP, Bayliss DA. Striatal cholinergic interneurons express a receptor-insensitive homomeric TASK-3-like background K+ current. J Neurophysiol. 2007;97:1546–1552. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
