Interaction between telencephalin and ERM family proteins mediates dendritic filopodia formation
- PMID: 17699668
- PMCID: PMC6672168
- DOI: 10.1523/JNEUROSCI.1047-07.2007
Interaction between telencephalin and ERM family proteins mediates dendritic filopodia formation
Abstract
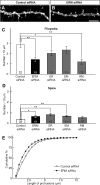
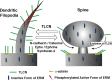
Dendritic filopodia are long, thin, actin-rich, and dynamic protrusions that are thought to play a critical role as a precursor of spines during neural development. We reported previously that a telencephalon-specific cell adhesion molecule, telencephalin (TLCN) [intercellular adhesion molecule-5 (ICAM-5)], is highly expressed in dendritic filopodia, facilitates the filopodia formation, and slows spine maturation. Here we demonstrate that TLCN cytoplasmic region binds ERM (ezrin/radixin/moesin) family proteins that link membrane proteins to actin cytoskeleton. In cultured hippocampal neurons, phosphorylated active forms of ERM proteins are colocalized with TLCN in dendritic filopodia, whereas alpha-actinin, another binding partner of TLCN, is colocalized with TLCN at surface membranes of soma and dendritic shafts. Expression of constitutively active ezrin induces dendritic filopodia formation, whereas small interference RNA-mediated knockdown of ERM proteins decreases filopodia density and accelerates spine maturation. These results indicate the important role of TLCN-ERM interaction in the formation of dendritic filopodia, which leads to subsequent synaptogenesis and establishment of functional neural circuitry in the developing brain.
Figures


References
-
- Balla T, Varnai P. Visualizing cellular phosphoinositide pools with GFP-fused protein-modules. Sci STKE. 2002;2002:L3. - PubMed
-
- Benson DL, Yoshihara Y, Mori K. Polarized distribution and cell type-specific localization of telencephalin, an intercellular adhesion molecule. J Neurosci Res. 1998;52:43–53. - PubMed
-
- Bretscher A, Edwards K, Fehon RG. ERM proteins and merlin: integrators at the cell cortex. Nat Rev Mol Cell Biol. 2002;3:586–599. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
