Protein kinase C regulates local synthesis and secretion of a neuropeptide required for activity-dependent long-term synaptic plasticity
- PMID: 17699674
- PMCID: PMC6672177
- DOI: 10.1523/JNEUROSCI.2322-07.2007
Protein kinase C regulates local synthesis and secretion of a neuropeptide required for activity-dependent long-term synaptic plasticity
Abstract
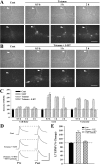
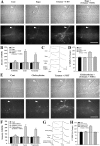
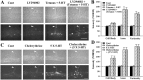
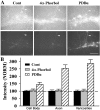
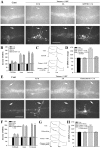
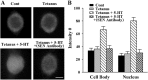
Long-term facilitation (LTF) of sensory neuron synapses in Aplysia is produced by either nonassociative or associative stimuli. Nonassociative LTF can be produced by five spaced applications of serotonin (5-HT) and requires a phosphoinosotide 3-kinase (PI3K)-dependent and rapamycin-sensitive increase in the local synthesis of the sensory neuron neuropeptide sensorin and a protein kinase A (PKA)-dependent increase in the secretion of the newly synthesized sensorin. We report here that associative LTF produced by a single pairing of a brief tetanus with one application of 5-HT requires a rapid protein kinase C (PKC)-dependent and rapamycin-sensitive increase in local sensorin synthesis. This rapid increase in sensorin synthesis does not require PI3K activity or the presence of the sensory neuron cell body but does require the presence of the motor neuron. The secretion of newly synthesized sensorin by 2 h after stimulation requires both PKA and PKC activities to produce associative LTF because incubation with exogenous anti-sensorin antibody or the kinase inhibitors after tetanus plus 5-HT blocked LTF. The secreted sensorin leads to phosphorylation and translocation of p42/44 mitogen-activated protein kinase (MAPK) into the nuclei of the sensory neurons. Thus, different stimuli activating different signaling pathways converge by regulating the synthesis and release of a neuropeptide to produce long-term synaptic plasticity.
Figures

Similar articles
-
Serotonin regulates the secretion and autocrine action of a neuropeptide to activate MAPK required for long-term facilitation in Aplysia.Neuron. 2004 Aug 5;43(3):373-85. doi: 10.1016/j.neuron.2004.07.011. Neuron. 2004. PMID: 15294145
-
Two signaling pathways regulate the expression and secretion of a neuropeptide required for long-term facilitation in Aplysia.J Neurosci. 2006 Jan 18;26(3):1026-35. doi: 10.1523/JNEUROSCI.4258-05.2006. J Neurosci. 2006. PMID: 16421322 Free PMC article.
-
Persistent long-term synaptic plasticity requires activation of a new signaling pathway by additional stimuli.J Neurosci. 2011 Jun 15;31(24):8841-50. doi: 10.1523/JNEUROSCI.1358-11.2011. J Neurosci. 2011. PMID: 21677168 Free PMC article.
-
Postsynaptic regulation of the development and long-term plasticity of Aplysia sensorimotor synapses in cell culture.J Neurobiol. 1994 Jun;25(6):666-93. doi: 10.1002/neu.480250608. J Neurobiol. 1994. PMID: 8071666 Review.
-
Cellular, molecular, and epigenetic mechanisms in non-associative conditioning: implications for pain and memory.Neurobiol Learn Mem. 2013 Oct;105:133-50. doi: 10.1016/j.nlm.2013.06.008. Epub 2013 Jun 22. Neurobiol Learn Mem. 2013. PMID: 23796633 Free PMC article. Review.
Cited by
-
Activity-dependent inhibitory gating in molecular signaling cascades induces a novel form of intermediate-term synaptic facilitation in Aplysia californica.Learn Mem. 2014 Mar 17;21(4):199-204. doi: 10.1101/lm.033894.113. Learn Mem. 2014. PMID: 24639486 Free PMC article.
-
Computational design of enhanced learning protocols.Nat Neurosci. 2011 Dec 25;15(2):294-7. doi: 10.1038/nn.2990. Nat Neurosci. 2011. PMID: 22197829 Free PMC article.
-
Multifunctional role of protein kinase C in regulating the formation and maturation of specific synapses.J Neurosci. 2007 Oct 24;27(43):11712-24. doi: 10.1523/JNEUROSCI.3305-07.2007. J Neurosci. 2007. PMID: 17959813 Free PMC article.
-
Persistent Associative Plasticity at an Identified Synapse Underlying Classical Conditioning Becomes Labile with Short-Term Homosynaptic Activation.J Neurosci. 2015 Dec 9;35(49):16159-70. doi: 10.1523/JNEUROSCI.2034-15.2015. J Neurosci. 2015. PMID: 26658867 Free PMC article.
-
Synaptic generation of an intracellular retrograde signal requires activation of the tyrosine kinase and mitogen-activated protein kinase signaling cascades in Aplysia.Neurobiol Learn Mem. 2015 Nov;125:47-54. doi: 10.1016/j.nlm.2015.07.017. Epub 2015 Jul 31. Neurobiol Learn Mem. 2015. PMID: 26238564 Free PMC article.
References
-
- Abrams TW, Yovell Y, Onyike CU, Cohen JE, Jarrard HE. Analysis of sequence-dependent interactions between transient calcium and transmitter stimuli in activating adenylyl cyclase in Aplysia: possible contribution to CS-US sequence requirement during conditioning. Learn Mem. 1998;4:496–509. - PubMed
-
- Alberini CM, Ghirardi M, Metz R, Kandel ER. C/EBP is an immediated-early gene required for the consolidation of long-term facilitation in Aplysia. Cell. 1994;76:1099–1114. - PubMed
-
- Bacskai BJ, Hochner B, Mahaut-Smith M, Adams SR, Kaang BK, Kandel ER, Tsien RY. Spatially resolved dynamics of cAMP and protein kinase A subunits in Aplysia sensory neurons. Science. 1993;260:222–226. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
