Timing and location of synaptic inputs determine modes of subthreshold integration in striatal medium spiny neurons
- PMID: 17699678
- PMCID: PMC6672187
- DOI: 10.1523/JNEUROSCI.2798-07.2007
Timing and location of synaptic inputs determine modes of subthreshold integration in striatal medium spiny neurons
Abstract
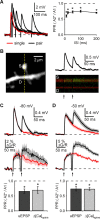
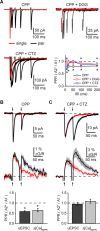
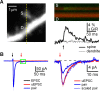
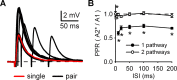
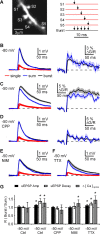
Medium spiny neurons (MSNs) are the principal cells of the striatum and perform a central role in sensorimotor processing. MSNs must integrate many excitatory inputs located across their dendrites to fire action potentials and enable striatal function. However, the dependence of synaptic responses on the temporal and spatial distribution of these inputs remains unknown. Here, we use whole-cell recordings, two-photon microscopy, and two-photon glutamate uncaging to examine subthreshold synaptic integration in MSNs from acute rat brain slices. We find that synaptic responses can summate sublinearly, linearly, or supralinearly depending on the spatiotemporal pattern of activity. Repetitive activity at single inputs leads to sublinear summation, reflecting long-lived AMPA receptor desensitization. In contrast, asynchronous activity at multiple inputs generates linear summation, with synapses on neighboring spines functioning independently. Finally, synchronous activity at multiple inputs triggers supralinear summation at depolarized potentials, reflecting activation of NMDA receptors and L-type calcium channels. Thus, the properties of subthreshold integration in MSNs are determined by the distribution of synaptic inputs and the differential activation of multiple postsynaptic conductances.
Figures



References
-
- Akopian G, Walsh JP. Corticostriatal paired-pulse potentiation produced by voltage-dependent activation of NMDA receptors and L-type Ca2+ channels. J Neurophysiol. 2002;87:157–165. - PubMed
-
- Alloway KD, Lou L, Nwabueze-Ogbo F, Chakrabarti S. Topography of cortical projections to the dorsolateral neostriatum in rats: multiple overlapping sensorimotor pathways. J Comp Neurol. 2006;499:33–48. - PubMed
-
- Bargas J, Ayala GX, Vilchis C, Pineda JC, Galarraga E. Ca2+-activated outward currents in neostriatal neurons. Neuroscience. 1999;88:479–488. - PubMed
-
- Cai X, Liang CW, Muralidharan S, Kao JP, Tang CM, Thompson SM. Unique roles of SK and Kv4.2 potassium channels in dendritic integration. Neuron. 2004;44:351–364. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
