The transcriptional coactivator Maml1 is required for Notch2-mediated marginal zone B-cell development
- PMID: 17699740
- PMCID: PMC2077311
- DOI: 10.1182/blood-2007-06-097030
The transcriptional coactivator Maml1 is required for Notch2-mediated marginal zone B-cell development
Abstract
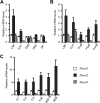
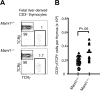
Signaling mediated by various Notch receptors and their ligands regulates diverse biological processes, including lymphoid cell fate decisions. Notch1 is required during T-cell development, while Notch2 and the Notch ligand Delta-like1 control marginal zone B (MZB) cell development. We previously determined that Mastermind-like (MAML) transcriptional coactivators are required for Notchinduced transcription by forming ternary nuclear complexes with Notch and the transcription factor CSL. The 3 MAML family members (MAML1-MAML3) are collectively essential for Notch activity in vivo, but whether individual MAMLs contribute to the specificity of Notch functions is unknown. Here, we addressed this question by studying lymphopoiesis in the absence of the Maml1 gene. Since Maml1(-/-) mice suffered perinatal lethality, hematopoietic chimeras were generated with Maml1(-/-), Maml1(+/-), or wild-type fetal liver progenitors. Maml1 deficiency minimally affected T-cell development, but was required for the development of MZB cells, similar to the phenotype of Notch2 deficiency. Moreover, the number of MZB cells correlated with Maml1 gene dosage. Since all 3 Maml genes were expressed in MZB cells and their precursors, these results suggest that Maml1 is specifically required for Notch2 signaling in MZB cells.
Figures



Similar articles
-
Mastermind critically regulates Notch-mediated lymphoid cell fate decisions.Blood. 2004 Sep 15;104(6):1696-702. doi: 10.1182/blood-2004-02-0514. Epub 2004 Jun 8. Blood. 2004. PMID: 15187027
-
Identification of a family of mastermind-like transcriptional coactivators for mammalian notch receptors.Mol Cell Biol. 2002 Nov;22(21):7688-700. doi: 10.1128/MCB.22.21.7688-7700.2002. Mol Cell Biol. 2002. PMID: 12370315 Free PMC article.
-
Cloning and functional characterization of the murine mastermind-like 1 (Maml1) gene.Gene. 2004 Mar 17;328:153-65. doi: 10.1016/j.gene.2003.12.007. Gene. 2004. PMID: 15019995
-
Transcriptional mechanisms by the coregulator MAML1.Curr Protein Pept Sci. 2009 Dec;10(6):570-6. doi: 10.2174/138920309789630543. Curr Protein Pept Sci. 2009. PMID: 19751190 Review.
-
Mastermind-like transcriptional co-activators: emerging roles in regulating cross talk among multiple signaling pathways.Oncogene. 2008 Sep 1;27(38):5138-47. doi: 10.1038/onc.2008.228. Oncogene. 2008. PMID: 18758483 Review.
Cited by
-
Single-cell RNA-seq mapping of chicken peripheral blood leukocytes.BMC Genomics. 2024 Jan 29;25(1):124. doi: 10.1186/s12864-024-10044-4. BMC Genomics. 2024. PMID: 38287279 Free PMC article.
-
Transitional B cells commit to marginal zone B cell fate by Taok3-mediated surface expression of ADAM10.Nat Immunol. 2017 Mar;18(3):313-320. doi: 10.1038/ni.3657. Epub 2017 Jan 9. Nat Immunol. 2017. PMID: 28068307
-
The follicular versus marginal zone B lymphocyte cell fate decision.Nat Rev Immunol. 2009 Nov;9(11):767-77. doi: 10.1038/nri2656. Nat Rev Immunol. 2009. PMID: 19855403 Review.
-
Olfactory Sensory Neurons Control Dendritic Complexity of Mitral Cells via Notch Signaling.PLoS Genet. 2016 Dec 27;12(12):e1006514. doi: 10.1371/journal.pgen.1006514. eCollection 2016 Dec. PLoS Genet. 2016. PMID: 28027303 Free PMC article.
-
Notch Signaling in B Cell Immune Responses.Front Immunol. 2021 Feb 5;11:609324. doi: 10.3389/fimmu.2020.609324. eCollection 2020. Front Immunol. 2021. PMID: 33613531 Free PMC article. Review.
References
-
- Maillard I, Fang T, Pear WS. Regulation of lymphoid development, differentiation, and function by the Notch pathway. Annu Rev Immunol. 2005;23:945–974. - PubMed
-
- Radtke F, Wilson A, Mancini SJ, MacDonald HR. Notch regulation of lymphocyte development and function. Nat Immunol. 2004;5:247–253. - PubMed
-
- Tanigaki K, Honjo T. Regulation of lymphocyte development by Notch signaling. Nat Immunol. 2007;8:451–456. - PubMed
-
- Kumano K, Chiba S, Kunisato A, et al. Notch1 but not Notch2 is essential for generating hematopoietic stem cells from endothelial cells. Immunity. 2003;18:699–711. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

