Dendritic cells are specialized accessory cells along with TGF- for the differentiation of Foxp3+ CD4+ regulatory T cells from peripheral Foxp3 precursors
- PMID: 17699744
- PMCID: PMC2234777
- DOI: 10.1182/blood-2007-05-088831
Dendritic cells are specialized accessory cells along with TGF- for the differentiation of Foxp3+ CD4+ regulatory T cells from peripheral Foxp3 precursors
Abstract
Foxp3(+)CD25(+)CD4(+) regulatory T cells are produced in the thymus (natural T regs) but can also differentiate from peripheral Foxp3(-)CD4(+) precursors (induced or adaptive T regs). We assessed antigen presenting cell (APC) requirements for the latter differentiation. With added transforming growth factor (TGF)-beta, both immature and mature populations of dendritic cells (DCs) induced antigen-specific Foxp3(+) T regs from Foxp3(-) precursors. Using endogenous TGF-beta, DCs from gut-associated mesenteric lymph nodes were capable of differentiating Foxp3(+)T regs. Spleen DCs were 100-fold more potent than DC-depleted APCs for the induction of T regs and required 10-fold lower doses of peptide antigen. Interleukin-2 (IL-2) was essential, but could be provided endogenously by T cells stimulated by DCs, but not other APCs. The required IL-2 was induced by DCs that expressed CD80/CD86 costimulatory molecules. The DC-induced Foxp3(+)T regs divided up to 6 times in 6 days and were comprised of CD62L and CD103 positive and negative forms. The induced Foxp3(+)T regs exerted suppression in vitro and blocked tumor immunity in vivo. These results indicate that DCs are specialized to differentiate functional peripheral Foxp3(+)T regs and help set the stage to use DCs to actively suppress the immune response in an antigen-specific manner.
Figures




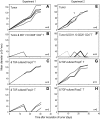
 indicates proliferating cells expressed Foxp3. (B) As in panel A, but CFSE-labeled DO11.10 RAG−/− Foxp3−CD25−CD4+ T cells were cultured with spleen CD11c+ DCs (2 × 104) or DC-depleted spleen cells (CD11c− APC, 2 × 105) at the indicated dose of peptide in the presence of TGF-β (2 ng/mL). Foxp3 expression and CFSE dilution were analyzed at day 6. Data are representative of 2 independent experiments.
indicates proliferating cells expressed Foxp3. (B) As in panel A, but CFSE-labeled DO11.10 RAG−/− Foxp3−CD25−CD4+ T cells were cultured with spleen CD11c+ DCs (2 × 104) or DC-depleted spleen cells (CD11c− APC, 2 × 105) at the indicated dose of peptide in the presence of TGF-β (2 ng/mL). Foxp3 expression and CFSE dilution were analyzed at day 6. Data are representative of 2 independent experiments.

Similar articles
-
CD8+ CD205+ splenic dendritic cells are specialized to induce Foxp3+ regulatory T cells.J Immunol. 2008 Nov 15;181(10):6923-33. doi: 10.4049/jimmunol.181.10.6923. J Immunol. 2008. PMID: 18981112 Free PMC article.
-
Crucial roles of B7-H1 and B7-DC expressed on mesenteric lymph node dendritic cells in the generation of antigen-specific CD4+Foxp3+ regulatory T cells in the establishment of oral tolerance.Blood. 2010 Sep 30;116(13):2266-76. doi: 10.1182/blood-2009-10-250472. Epub 2010 Jun 23. Blood. 2010. PMID: 20574047 Free PMC article.
-
Dendritic cells with TGF-beta1 differentiate naive CD4+CD25- T cells into islet-protective Foxp3+ regulatory T cells.Proc Natl Acad Sci U S A. 2007 Feb 20;104(8):2821-6. doi: 10.1073/pnas.0611646104. Epub 2007 Feb 16. Proc Natl Acad Sci U S A. 2007. PMID: 17307871 Free PMC article.
-
Dendritic cells expand antigen-specific Foxp3+ CD25+ CD4+ regulatory T cells including suppressors of alloreactivity.Immunol Rev. 2006 Aug;212:314-29. doi: 10.1111/j.0105-2896.2006.00422.x. Immunol Rev. 2006. PMID: 16903923 Review.
-
Dendritic cells and (CD4+)CD25+ T regulatory cells: crosstalk between two professionals in immunity versus tolerance.Front Biosci. 2006 May 1;11:1360-70. doi: 10.2741/1889. Front Biosci. 2006. PMID: 16368522 Review.
Cited by
-
Mature dendritic cells enriched in regulatory molecules may control regulatory T cells and the prognosis of head and neck cancer.Cancer Sci. 2023 Apr;114(4):1256-1269. doi: 10.1111/cas.15698. Epub 2023 Jan 23. Cancer Sci. 2023. PMID: 36529525 Free PMC article.
-
The extrinsic RNA-sensing pathway for adjuvant immunotherapy of cancer.Cancer Immunol Immunother. 2009 Aug;58(8):1175-84. doi: 10.1007/s00262-008-0652-9. Epub 2009 Jan 31. Cancer Immunol Immunother. 2009. PMID: 19184005 Free PMC article. Review.
-
Immune regulation by non-lymphoid cells in transplantation.Clin Exp Immunol. 2009 Apr;156(1):25-34. doi: 10.1111/j.1365-2249.2009.03877.x. Epub 2009 Jan 22. Clin Exp Immunol. 2009. PMID: 19196251 Free PMC article. Review.
-
Distinct subpopulations of epithelial ovarian cancer cells can differentially induce macrophages and T regulatory cells toward a pro-tumor phenotype.Am J Reprod Immunol. 2012 Mar;67(3):256-65. doi: 10.1111/j.1600-0897.2011.01068.x. Epub 2011 Sep 14. Am J Reprod Immunol. 2012. PMID: 21917055 Free PMC article.
-
Recent progress in understanding the phenotype and function of intestinal dendritic cells and macrophages.Mucosal Immunol. 2008 Nov;1(6):460-9. doi: 10.1038/mi.2008.61. Epub 2008 Sep 17. Mucosal Immunol. 2008. PMID: 19079213 Free PMC article. Review.
References
-
- O'Garra A. Cytokines induce the development of functionally heterogeneous T helper cell subsets. Immunity. 1998;8:275–283. - PubMed
-
- Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–793. - PubMed
-
- Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–476. - PubMed
-
- Weaver CT, Harrington LE, Mangan PR, Gavrieli M, Murphy KM. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity. 2006;24:677–688. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

