Angiomotin regulates endothelial cell migration during embryonic angiogenesis
- PMID: 17699752
- PMCID: PMC1948860
- DOI: 10.1101/gad.432007
Angiomotin regulates endothelial cell migration during embryonic angiogenesis
Abstract
The development of the embryonic vascular system into a highly ordered network requires precise control over the migration and branching of endothelial cells (ECs). We have previously identified angiomotin (Amot) as a receptor for the angiogenesis inhibitor angiostatin. Furthermore, DNA vaccination targeting Amot inhibits angiogenesis and tumor growth. However, little is known regarding the role of Amot in physiological angiogenesis. We therefore investigated the role of Amot in embryonic neovascularization during zebrafish and mouse embryogenesis. Here we report that knockdown of Amot in zebrafish reduced the number of filopodia of endothelial tip cells and severely impaired the migration of intersegmental vessels. We further show that 75% of Amot knockout mice die between embryonic day 11 (E11) and E11.5 and exhibit severe vascular insufficiency in the intersomitic region as well as dilated vessels in the brain. Furthermore, using ECs differentiated from embryonic stem (ES) cells, we demonstrate that Amot-deficient cells have intact response to vascular endothelial growth factor (VEGF) in regard to differentiation and proliferation. However, the chemotactic response to VEGF was abolished in Amot-deficient cells. We provide evidence that Amot is important for endothelial polarization during migration and that Amot controls Rac1 activity in endothelial and epithelial cells. Our data demonstrate a critical role for Amot during vascular patterning and endothelial polarization.
Figures

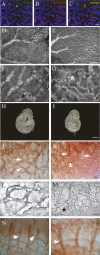
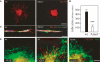




References
-
- Aepfelbacher M., Essler M., Huber E., Sugai M., Weber P.C., Essler M., Huber E., Sugai M., Weber P.C., Huber E., Sugai M., Weber P.C., Sugai M., Weber P.C., Weber P.C. Bacterial toxins block endothelial wound repair. Evidence that Rho GTPases control cytoskeletal rearrangements in migrating endothelial cells. Arterioscler. Thromb. Vasc. Biol. 1997;17:1623–1629. - PubMed
-
- Balconi G., Spagnuolo R., Dejana E., Spagnuolo R., Dejana E., Dejana E. Development of endothelial cell lines from embryonic stem cells: A tool for studying genetically manipulated endothelial cells in vitro. Arterioscler. Thromb. Vasc. Biol. 2000;20:1443–1451. - PubMed
-
- Berglin L., Sarman S., van der Ploeg I., Steen B., Ming Y., Itohara S., Seregard S., Kvanta A., Sarman S., van der Ploeg I., Steen B., Ming Y., Itohara S., Seregard S., Kvanta A., van der Ploeg I., Steen B., Ming Y., Itohara S., Seregard S., Kvanta A., Steen B., Ming Y., Itohara S., Seregard S., Kvanta A., Ming Y., Itohara S., Seregard S., Kvanta A., Itohara S., Seregard S., Kvanta A., Seregard S., Kvanta A., Kvanta A. Reduced choroidal neovascular membrane formation in matrix metalloproteinase-2-deficient mice. Invest. Ophthalmol. Vis. Sci. 2003;44:403–408. - PubMed
-
- Bostrom H., Willetts K., Pekny M., Leveen P., Lindahl P., Hedstrand H., Pekna M., Hellstrom M., Gebre-Medhin S., Schalling M., Willetts K., Pekny M., Leveen P., Lindahl P., Hedstrand H., Pekna M., Hellstrom M., Gebre-Medhin S., Schalling M., Pekny M., Leveen P., Lindahl P., Hedstrand H., Pekna M., Hellstrom M., Gebre-Medhin S., Schalling M., Leveen P., Lindahl P., Hedstrand H., Pekna M., Hellstrom M., Gebre-Medhin S., Schalling M., Lindahl P., Hedstrand H., Pekna M., Hellstrom M., Gebre-Medhin S., Schalling M., Hedstrand H., Pekna M., Hellstrom M., Gebre-Medhin S., Schalling M., Pekna M., Hellstrom M., Gebre-Medhin S., Schalling M., Hellstrom M., Gebre-Medhin S., Schalling M., Gebre-Medhin S., Schalling M., Schalling M., et al. PDGF-A signaling is a critical event in lung alveolar myofibroblast development and alveogenesis. Cell. 1996;85:863–873. - PubMed
-
- Bratt A., Wilson W.J., Troyanovsky B., Aase K., Kessler R., Van Meir E.G., Holmgren L., Wilson W.J., Troyanovsky B., Aase K., Kessler R., Van Meir E.G., Holmgren L., Troyanovsky B., Aase K., Kessler R., Van Meir E.G., Holmgren L., Aase K., Kessler R., Van Meir E.G., Holmgren L., Kessler R., Van Meir E.G., Holmgren L., Van Meir E.G., Holmgren L., Holmgren L. Angiomotin belongs to a novel protein family with conserved coiled-coil and PDZ binding domains. Gene. 2002;298:69–77. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
