Immunogenicity of a fourth dose of Haemophilus influenzae type b (Hib) conjugate vaccine and antibody persistence in young children from the United Kingdom who were primed with acellular or whole-cell pertussis component-containing Hib combinations in infancy
- PMID: 17699835
- PMCID: PMC2168123
- DOI: 10.1128/CVI.00191-07
Immunogenicity of a fourth dose of Haemophilus influenzae type b (Hib) conjugate vaccine and antibody persistence in young children from the United Kingdom who were primed with acellular or whole-cell pertussis component-containing Hib combinations in infancy
Abstract
In response to the rising incidence of Haemophilus influenzae type b (Hib) disease in the United Kingdom, a national campaign to give a booster dose of single-antigen Hib conjugate vaccine to children aged 6 months to 4 years was undertaken in 2003. Children (n = 386) eligible for Hib vaccine in the campaign were recruited. Hib antibody concentrations were measured before boost and at 1 month, 6 months, 1 year, and 2 years after boost and were analyzed according to children's ages at booster dose and whether a Hib combination vaccine containing acellular pertussis (aP) or whole-cell pertussis (wP) components was given in infancy. The geometric mean antibody concentrations (GMCs) before the booster declined as the time since primary immunization increased (P < 0.001), and GMCs were threefold higher in recipients of wP-Hib than aP-Hib combination vaccines (P < 0.001). GMCs 1 month after the booster increased with age (P < 0.001) as follows: 6 to 11 months; 30 microg/ml (95% confidence interval [CI], 22 to 40); 12 to 17 months, 68 microg/ml (95% CI, 38 to 124); and 2 to 4 years, 182 microg/ml (151 to 220), with no difference according to the type of priming vaccine received. Antibody levels declined after the booster, but 2 years later, GMCs were more than 1.0 microg/ml for all age groups. By extrapolating data for the decline in antibody levels, we found the GMCs 4 years after boosting were predicted to be 0.6, 1.4, and 2.6 microg/ml for those boosted at 6 to 11 months, 12 to 17 months, and 2 to 4 years, respectively, with levels of at least 0.15 microg/ml in about 90% of individuals. A booster dose of Hib vaccine given after the first year of life should provide long-lasting protection.
Figures
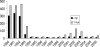
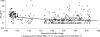
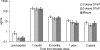
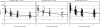
Similar articles
-
Acellular pertussis vaccine at birth and one month induces antibody responses by two months of age.Pediatr Infect Dis J. 2010 Mar;29(3):209-15. doi: 10.1097/INF.0b013e3181bc98d5. Pediatr Infect Dis J. 2010. PMID: 20009964 Clinical Trial.
-
Persistence of antibody response following a booster dose of Hib-MenC-TT glycoconjugate vaccine to five years: a follow-up study.Pediatr Infect Dis J. 2012 Oct;31(10):1069-73. doi: 10.1097/INF.0b013e318262528c. Pediatr Infect Dis J. 2012. PMID: 22673139 Clinical Trial.
-
Antibody concentration and clinical protection after Hib conjugate vaccination in the United Kingdom.JAMA. 2000 Nov 8;284(18):2334-40. doi: 10.1001/jama.284.18.2334. JAMA. 2000. PMID: 11066183
-
Two decades of experience with the Haemophilus influenzae serotype b conjugate vaccine in the United Kingdom.Clin Ther. 2012 Feb;34(2):385-99. doi: 10.1016/j.clinthera.2011.11.027. Epub 2012 Jan 12. Clin Ther. 2012. PMID: 22244051 Review.
-
Immunogenicity of routinely used childhood vaccines when coadministered with the 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV).Pediatr Infect Dis J. 2009 Apr;28(4 Suppl):S97-S108. doi: 10.1097/INF.0b013e318199f61b. Pediatr Infect Dis J. 2009. PMID: 19325452 Review.
Cited by
-
The effectiveness of conjugate Haemophilus influenzae type B vaccine in The Gambia 14 years after introduction.Clin Infect Dis. 2013 Dec;57(11):1527-34. doi: 10.1093/cid/cit598. Epub 2013 Sep 17. Clin Infect Dis. 2013. PMID: 24046305 Free PMC article.
-
Naturally acquired and conjugate vaccine-induced antibody to Haemophilus influenzae type b (Hib) polysaccharide in Malian children: serological assessment of the Hib immunization program in Mali.Am J Trop Med Hyg. 2012 Jun;86(6):1026-31. doi: 10.4269/ajtmh.2012.11-0807. Am J Trop Med Hyg. 2012. PMID: 22665612 Free PMC article.
-
Lagging Immune Response to Haemophilus influenzae Serotype b (Hib) Conjugate Vaccine after the Primary Vaccination with Hib of Infants in The Netherlands.Vaccines (Basel). 2020 Jun 30;8(3):347. doi: 10.3390/vaccines8030347. Vaccines (Basel). 2020. PMID: 32629935 Free PMC article.
-
Kinetics of antibody persistence following administration of a combination meningococcal serogroup C and haemophilus influenzae type b conjugate vaccine in healthy infants in the United Kingdom primed with a monovalent meningococcal serogroup C vaccine.Clin Vaccine Immunol. 2010 Jan;17(1):154-9. doi: 10.1128/CVI.00384-09. Epub 2009 Nov 11. Clin Vaccine Immunol. 2010. PMID: 19906895 Free PMC article.
-
Maintaining protection against invasive bacteria with protein-polysaccharide conjugate vaccines.Nat Rev Immunol. 2009 Mar;9(3):213-20. doi: 10.1038/nri2494. Nat Rev Immunol. 2009. PMID: 19214194
References
-
- Anderson, P., D. L. Ingram, M. E. Pichichero, and G. Peter. 2000. A high degree of natural immunologic priming to the capsular polysaccharide may not prevent Haemophilus influenzae type b meningitis. Pediatr. Infect. Dis. J. 19:589-591. - PubMed
-
- Barbour, M. L., R. T. Mayon-White, C. Coles, D. W. Crook, and E. R. Moxon. 1995. The impact of conjugate vaccine on carriage of Haemophilus influenzae type b. J. Infect. Dis. 171:93-98. - PubMed
-
- Bernasconi, N. L., E. Traggiai, and A. Lanzavecchia. 2002. Maintenance of serological memory by polyclonal activation of human memory B cells. Science 298:2199-2202. - PubMed
-
- Booy, R., S. Hodgson, L. Carpenter, R. Mayon-White, M. Slack, J. Macfarlane, E. A. Haworth, M. Kiddle, S. Shribman, and J. S. Roberts. 1994. Efficacy of Haemophilus influenzae type b conjugate vaccine PRP-T. Lancet 344:362-366. - PubMed
-
- Eskola, J., J. Ward, R. Dagan, D. Goldblatt, F. Zepp, and C. A. Siegrist. 1999. Combined vaccination of Haemophilus influenzae type b conjugate and diphtheria-tetanus-pertussis containing acellular pertussis. Lancet 354:2063-2068. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

