Nitric oxide selectively depletes macrophages in atherosclerotic plaques via induction of endoplasmic reticulum stress
- PMID: 17700714
- PMCID: PMC2050816
- DOI: 10.1038/sj.bjp.0707426
Nitric oxide selectively depletes macrophages in atherosclerotic plaques via induction of endoplasmic reticulum stress
Abstract
Background and purpose: Macrophages in atherosclerotic plaques have a tremendous impact on atherogenesis and plaque destabilization. We previously demonstrated that treatment of plaques in cholesterol-fed rabbits with the nitric oxide (NO) donor molsidomine preferentially eliminates macrophages, thereby favouring features of plaque stability. In this study, we investigated the underlying mechanism.
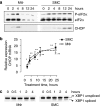
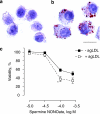
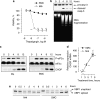
Experimental approach: Macrophages and smooth muscle cells (SMCs) were treated in vitro with the NO donors, spermine NONOate or S-nitroso-N-acetylpenicillamine (SNAP) as well as with the well-known endoplasmic reticulum (ER) stress inducers thapsigargin, tunicamycin, dithiothreitol or brefeldin A. Cell viability was analysed by Neutral Red viability assays. Cleavage of caspase-3, DNA fragmentation and ultrastructural changes were examined to characterize the type of macrophage death. Induction of ER stress was evaluated by measuring C/EBP homologous protein (CHOP) expression, phosphorylation of eukaryotic initiation factor 2 alpha (eIF2a), splicing of X-box binding protein 1 (XBP1) and inhibition of protein synthesis.
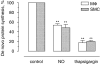
Key results: Macrophages and SMCs treated with spermine NONOate or SNAP showed several signs of ER stress, including upregulation of CHOP expression, hyperphosphorylation of eIF2 alpha, inhibition of de novo protein synthesis and splicing of XBP1 mRNA. These effects were similar in macrophages and SMCs, yet only macrophages underwent apoptosis. Plaques from molsidomine-treated atherosclerotic rabbits showed a 2.7-fold increase in CHOP expression as compared to placebo. Beside NO, selective induction of macrophage death could be initiated with thapsigargin and tunicamycin.
Conclusions and implications: Induction of ER stress explains selective depletion of macrophages in atherosclerotic plaques by a NO donor, probably via inhibition of protein synthesis.
Figures
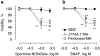

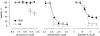
Similar articles
-
The endoplasmic reticulum stress-C/EBP homologous protein pathway-mediated apoptosis in macrophages contributes to the instability of atherosclerotic plaques.Arterioscler Thromb Vasc Biol. 2010 Oct;30(10):1925-32. doi: 10.1161/ATVBAHA.110.206094. Epub 2010 Jul 22. Arterioscler Thromb Vasc Biol. 2010. PMID: 20651282
-
Nitric oxide-induced apoptosis in pancreatic beta cells is mediated by the endoplasmic reticulum stress pathway.Proc Natl Acad Sci U S A. 2001 Sep 11;98(19):10845-50. doi: 10.1073/pnas.191207498. Epub 2001 Aug 28. Proc Natl Acad Sci U S A. 2001. PMID: 11526215 Free PMC article.
-
Effect of nitric oxide on endoplasmic reticulum calcium homeostasis, protein synthesis and energy metabolism.Cell Calcium. 2000 Feb;27(2):107-15. doi: 10.1054/ceca.1999.0099. Cell Calcium. 2000. PMID: 10756977
-
Selective depletion of macrophages in atherosclerotic plaques via macrophage-specific initiation of cell death.Trends Cardiovasc Med. 2007 Feb;17(2):69-75. doi: 10.1016/j.tcm.2006.12.004. Trends Cardiovasc Med. 2007. PMID: 17292050 Review.
-
Selective removal of macrophages in atherosclerotic plaques as a pharmacological approach for plaque stabilization: benefits versus potential complications.Curr Vasc Pharmacol. 2010 Jul;8(4):495-508. doi: 10.2174/157016110791330816. Curr Vasc Pharmacol. 2010. PMID: 19485918 Review.
Cited by
-
Pharmacological modulation of cell death in atherosclerosis: a promising approach towards plaque stabilization?Br J Pharmacol. 2011 Sep;164(1):1-13. doi: 10.1111/j.1476-5381.2011.01342.x. Br J Pharmacol. 2011. PMID: 21418184 Free PMC article. Review.
-
Mouse aorta-derived mesenchymal progenitor cells contribute to and enhance the immune response of macrophage cells under inflammatory conditions.Stem Cell Res Ther. 2015 Apr 14;6(1):56. doi: 10.1186/s13287-015-0071-8. Stem Cell Res Ther. 2015. PMID: 25889992 Free PMC article.
-
Molecular Mechanism Underlying Role of the XBP1s in Cardiovascular Diseases.J Cardiovasc Dev Dis. 2022 Dec 14;9(12):459. doi: 10.3390/jcdd9120459. J Cardiovasc Dev Dis. 2022. PMID: 36547457 Free PMC article. Review.
-
Macrophage Death as a Pharmacological Target in Atherosclerosis.Front Pharmacol. 2019 Apr 4;10:306. doi: 10.3389/fphar.2019.00306. eCollection 2019. Front Pharmacol. 2019. PMID: 31019462 Free PMC article. Review.
-
Effects of emotional and physiological stress on plaque instability in apolipoprotein E knockout mice.J Physiol Biochem. 2011 Sep;67(3):401-13. doi: 10.1007/s13105-011-0090-6. Epub 2011 Apr 22. J Physiol Biochem. 2011. PMID: 21512836
References
-
- Berridge MJ. The endoplasmic reticulum: a multifunctional signaling organelle. Cell Calcium. 2002;32:235–249. - PubMed
-
- Bjornheden T, Bondjers G. Oxygen consumption in aortic tissue from rabbits with diet-induced atherosclerosis. Arteriosclerosis. 1987;7:238–247. - PubMed
-
- Clarke MC, Figg N, Maguire JJ, Davenport AP, Goddard M, Littlewood TD, et al. Apoptosis of vascular smooth muscle cells induces features of plaque vulnerability in atherosclerosis. Nat Med. 2006;12:1075–1080. - PubMed
-
- Croons V, Martinet W, Herman AG, Timmermans J-P, De Meyer GRY. Selective clearance of macrophages in atherosclerotic plaques by the protein synthesis inhibitor cycloheximide. J Pharmacol Exp Ther. 2007;320:986–993. - PubMed
-
- De Meyer GRY, Kockx MM, Knaapen MWM, Martinet W, De Cleen DMM, Bult H, et al. Nitric oxide donor molsidomine favors features of atherosclerotic plaque stability during cholesterol lowering in rabbits. J Cardiovasc Pharmacol. 2003;41:970–978. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

