Evidence of molecular alignment fluctuations in aqueous dilute liquid crystalline media
- PMID: 17701275
- PMCID: PMC2039844
- DOI: 10.1007/s10858-007-9182-6
Evidence of molecular alignment fluctuations in aqueous dilute liquid crystalline media
Abstract
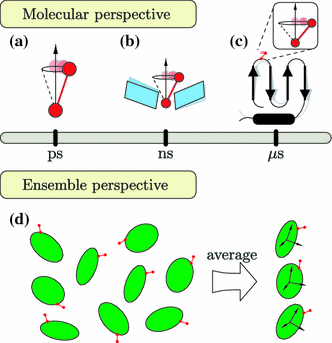
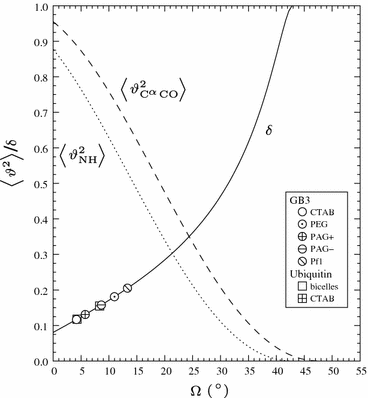
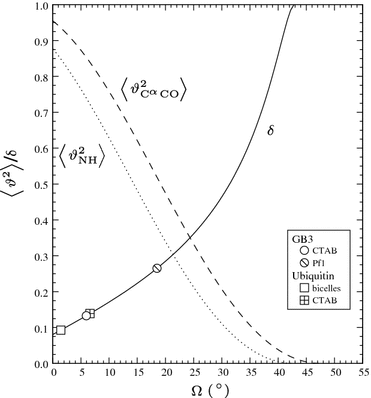
Protein dynamics can be studied by NMR measurements of aqueous dilute liquid crystalline samples. However, the measured residual dipolar couplings are sensitive not only to internal fluctuations but to all changes in internuclear vectors relative to the laboratory frame. We show that side-chain fluctuations and bond librations in the ps-ns time scale perturb the molecular shape and charge distribution of a small globular protein sufficiently to cause a noticeable variation in the molecular alignment. The alignment variation disperses the bond vectors of a conformational ensemble even further from the dispersion already caused by internal fluctuations of a protein. Consequently RDC-probed order parameters are lower than those obtained by laboratory frame relaxation measurements.
Figures
Similar articles
-
Conformational fluctuations affect protein alignment in dilute liquid crystal media.J Am Chem Soc. 2006 Apr 5;128(13):4371-6. doi: 10.1021/ja0576334. J Am Chem Soc. 2006. PMID: 16569014
-
Influence of the fluctuations of the alignment tensor on the analysis of the structure and dynamics of proteins using residual dipolar couplings.J Biomol NMR. 2008 Jan;40(1):71-81. doi: 10.1007/s10858-007-9210-6. Epub 2007 Nov 21. J Biomol NMR. 2008. PMID: 18030429
-
Conformational flexibility of a microcrystalline globular protein: order parameters by solid-state NMR spectroscopy.J Am Chem Soc. 2006 Sep 6;128(35):11505-12. doi: 10.1021/ja062443u. J Am Chem Soc. 2006. PMID: 16939274
-
Weak alignment NMR: a hawk-eyed view of biomolecular structure.Curr Opin Struct Biol. 2005 Oct;15(5):563-70. doi: 10.1016/j.sbi.2005.08.006. Curr Opin Struct Biol. 2005. PMID: 16140525 Review.
-
Weak alignment offers new NMR opportunities to study protein structure and dynamics.Protein Sci. 2003 Jan;12(1):1-16. doi: 10.1110/ps.0233303. Protein Sci. 2003. PMID: 12493823 Free PMC article. Review.
Cited by
-
CoNSEnsX: an ensemble view of protein structures and NMR-derived experimental data.BMC Struct Biol. 2010 Oct 29;10:39. doi: 10.1186/1472-6807-10-39. BMC Struct Biol. 2010. PMID: 21034466 Free PMC article.
-
Self-consistent residual dipolar coupling based model-free analysis for the robust determination of nanosecond to microsecond protein dynamics.J Biomol NMR. 2008 Jul;41(3):139-55. doi: 10.1007/s10858-008-9244-4. Epub 2008 Jun 4. J Biomol NMR. 2008. PMID: 18523727 Free PMC article.
-
Assessing and refining molecular dynamics simulations of proteins with nuclear magnetic resonance data.Biophys Rev. 2012 Sep;4(3):189-203. doi: 10.1007/s12551-012-0087-6. Epub 2012 Sep 1. Biophys Rev. 2012. PMID: 28510078 Free PMC article. Review.
References
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1016/j.sbi.2005.08.006', 'is_inner': False, 'url': 'https://doi.org/10.1016/j.sbi.2005.08.006'}, {'type': 'PubMed', 'value': '16140525', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/16140525/'}]}
- Bax A, Grishaev A (2005) Weak alignment NMR: a hawk-eyed view of biomolecular structure. Curr Opin Struct Biol 15:563–570 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'PubMed', 'value': '11462810', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/11462810/'}]}
- Bax A, Kontaxis G, Tjandra N (2001) Dipolar couplings in macromolecular structure determination. Methods Enzymol 339:127–174 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1021/ja048785m', 'is_inner': False, 'url': 'https://doi.org/10.1021/ja048785m'}, {'type': 'PubMed', 'value': '15212507', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/15212507/'}]}
- Bernadó P, Blackledge M (2004) Local dynamic amplitudes on the protein backbone from dipolar couplings: toward the elucidation of slower motions in biomolecules. J Am Chem Soc 126:7760–7761 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1021/ja055538p', 'is_inner': False, 'url': 'https://doi.org/10.1021/ja055538p'}, {'type': 'PubMed', 'value': '16366524', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/16366524/'}]}
- Bernadó P, Bertoncini CW, Griesinger C, Zweckstetter M, Blackledge M (2005) Defining long-range order and local disorder in native α-Synuclein using residual dipolar couplings. J Am Chem Soc 127:17968–17969 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1021/ja000858o', 'is_inner': False, 'url': 'https://doi.org/10.1021/ja000858o'}]}
- Bewley CA, Clore GM (2000) Determination of the relative orientation of the two halves of the domain-swapped dimer of cyanovirin-N in solution using dipolar couplings and rigid body minimization. J Am Chem Soc 122:6009–6016
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

