Oral curcumin mitigates the clinical and neuropathologic phenotype of the Trembler-J mouse: a potential therapy for inherited neuropathy
- PMID: 17701891
- PMCID: PMC1950845
- DOI: 10.1086/519926
Oral curcumin mitigates the clinical and neuropathologic phenotype of the Trembler-J mouse: a potential therapy for inherited neuropathy
Abstract
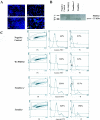
Mutations in myelin genes cause inherited peripheral neuropathies that range in severity from adult-onset Charcot-Marie-Tooth disease type 1 to childhood-onset Dejerine-Sottas neuropathy and congenital hypomyelinating neuropathy. Many myelin gene mutants that cause severe disease, such as those in the myelin protein zero gene (MPZ) and the peripheral myelin protein 22 gene (PMP22), appear to make aberrant proteins that accumulate primarily within the endoplasmic reticulum (ER), resulting in Schwann cell death by apoptosis and, subsequently, peripheral neuropathy. We previously showed that curcumin supplementation could abrogate ER retention and aggregation-induced apoptosis associated with neuropathy-causing MPZ mutants. We now show reduced apoptosis after curcumin treatment of cells in tissue culture that express PMP22 mutants. Furthermore, we demonstrate that oral administration of curcumin partially mitigates the severe neuropathy phenotype of the Trembler-J mouse model in a dose-dependent manner. Administration of curcumin significantly decreases the percentage of apoptotic Schwann cells and results in increased number and size of myelinated axons in sciatic nerves, leading to improved motor performance. Our findings indicate that curcumin treatment is sufficient to relieve the toxic effect of mutant aggregation-induced apoptosis and improves the neuropathologic phenotype in an animal model of human neuropathy, suggesting a potential therapeutic role in selected forms of inherited peripheral neuropathies.
Figures







Similar articles
-
Curcumin treatment abrogates endoplasmic reticulum retention and aggregation-induced apoptosis associated with neuropathy-causing myelin protein zero-truncating mutants.Am J Hum Genet. 2005 Nov;77(5):841-50. doi: 10.1086/497541. Epub 2005 Sep 30. Am J Hum Genet. 2005. PMID: 16252242 Free PMC article.
-
Curcumin facilitates a transitory cellular stress response in Trembler-J mice.Hum Mol Genet. 2013 Dec 1;22(23):4698-705. doi: 10.1093/hmg/ddt318. Epub 2013 Jul 11. Hum Mol Genet. 2013. PMID: 23847051 Free PMC article.
-
Curcumin derivatives promote Schwann cell differentiation and improve neuropathy in R98C CMT1B mice.Brain. 2012 Dec;135(Pt 12):3551-66. doi: 10.1093/brain/aws299. Brain. 2012. PMID: 23250879 Free PMC article.
-
Charcot-Marie-Tooth disease and related inherited neuropathies.Medicine (Baltimore). 1996 Sep;75(5):233-50. doi: 10.1097/00005792-199609000-00001. Medicine (Baltimore). 1996. PMID: 8862346 Review.
-
Mutations in the peripheral myelin genes and associated genes in inherited peripheral neuropathies.Hum Mutat. 1999;13(1):11-28. doi: 10.1002/(SICI)1098-1004(1999)13:1<11::AID-HUMU2>3.0.CO;2-A. Hum Mutat. 1999. PMID: 9888385 Review.
Cited by
-
Charcot-Marie-Tooth disease.Curr Treat Options Neurol. 2008 Mar;10(2):94-102. doi: 10.1007/s11940-008-0011-3. Curr Treat Options Neurol. 2008. PMID: 18334132
-
Development and Validation of the Pediatric Charcot-Marie-Tooth Disease Quality of Life Outcome Measure.Ann Neurol. 2021 Feb;89(2):369-379. doi: 10.1002/ana.25966. Epub 2020 Dec 7. Ann Neurol. 2021. PMID: 33222249 Free PMC article.
-
Rescue of photoreceptor degeneration by curcumin in transgenic rats with P23H rhodopsin mutation.PLoS One. 2011;6(6):e21193. doi: 10.1371/journal.pone.0021193. Epub 2011 Jun 29. PLoS One. 2011. PMID: 21738619 Free PMC article.
-
Quantitative proteomics unveils known and previously unrecognized alterations in neuropathic nerves.J Neurochem. 2024 Sep;168(9):3154-3170. doi: 10.1111/jnc.16189. Epub 2024 Jul 29. J Neurochem. 2024. PMID: 39072727
-
The PMP22 gene and its related diseases.Mol Neurobiol. 2013 Apr;47(2):673-98. doi: 10.1007/s12035-012-8370-x. Epub 2012 Dec 7. Mol Neurobiol. 2013. PMID: 23224996 Free PMC article. Review.
References
Web Resources
-
- Inherited Peripheral Neuropathies Mutation Database, http://www.molgen.ua.ac.be/CMTMutations/
-
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for CMT1, CMT2, HNPP, DSN, and CHN)
References
-
- Skre H (1974) Genetic and clinical aspects of Charcot-Marie-Tooth’s disease. Clin Genet 6:98 - PubMed
-
- Lupski JR, Garcia CA (2001) Charcoat-Marie-Tooth peripheral neuropathies and related disorders. In: Scriver CR, Beaudet AL, Sly WS, Valle D (eds) The metabolic and molecular basis of inherited diseases. McGraw-Hill, New York, pp 5759–5788
-
- Shy ME, Lupski JR, Chance PF, Klein CJ, Dyck PJ (2005) Hereditary motor and sensory neuropathies: an overview of clinical, genetic, electrophysiologic, and pathologic features. In: Dyck PJ, Thomas PK (eds) Peripheral neuropathy. Elsevier Saunders, Philadelphia, pp 1623–1658
-
- Timmerman V, Lupski JR, De Jonghe P (2006) Molecular genetics, biology, and therapy for inherited peripheral neuropathies. Neuromolecular medicine special issue. Humana Press, Totowa, NJ, pp 1–278 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

