Arts syndrome is caused by loss-of-function mutations in PRPS1
- PMID: 17701896
- PMCID: PMC1950830
- DOI: 10.1086/520706
Arts syndrome is caused by loss-of-function mutations in PRPS1
Abstract
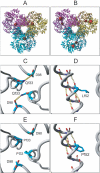
Arts syndrome is an X-linked disorder characterized by mental retardation, early-onset hypotonia, ataxia, delayed motor development, hearing impairment, and optic atrophy. Linkage analysis in a Dutch family and an Australian family suggested that the candidate gene maps to Xq22.1-q24. Oligonucleotide microarray expression profiling of fibroblasts from two probands of the Dutch family revealed reduced expression levels of the phosphoribosyl pyrophosphate synthetase 1 gene (PRPS1). Subsequent sequencing of PRPS1 led to the identification of two different missense mutations, c.455T-->C (p.L152P) in the Dutch family and c.398A-->C (p.Q133P) in the Australian family. Both mutations result in a loss of phosphoribosyl pyrophosphate synthetase 1 activity, as was shown in silico by molecular modeling and was shown in vitro by phosphoribosyl pyrophosphate synthetase activity assays in erythrocytes and fibroblasts from patients. This is in contrast to the gain-of-function mutations in PRPS1 that were identified previously in PRPS-related gout. The loss-of-function mutations of PRPS1 likely result in impaired purine biosynthesis, which is supported by the undetectable hypoxanthine in urine and the reduced uric acid levels in serum from patients. To replenish low levels of purines, treatment with S-adenosylmethionine theoretically could have therapeutic efficacy, and a clinical trial involving the two affected Australian brothers is currently underway.
Figures


References
Web Resources
-
- GenBank, http://www.ncbi.nlm.nih.gov/Genbank/ (for accession numbers NM_003588.3, NM_002764.2, and NM_012471.2 and those in tables 1 and 3)
-
- NCBI Map Viewer, http://www.ncbi.nlm.nih.gov/mapview/ (for build 36.2)
-
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for PRPS1, PRPS2, PRPS-related gout, orotic aciduria, Arts syndrome, HPRT1 deficiency, PNP deficiency, GUSB, CUL4B, TRPC5, GJB2, ACSL4, AGTR2, PAK3,UBE2A, C1GALT1C1, PLS3, IL13RA1, Lesch-Nyhan syndrome, and adenosine deaminase deficiency)
-
- RCSB Protein Data Bank, http://www.pdb.org/
References
-
- Becker MA (2001) Phosphoribosylpyrophosphate synthetase and the regulation of phosphoribosylpyrophosphate production in human cells. Prog Nucleic Acid Res Mol Biol 69:115–148 - PubMed
-
- Becker MA, Heidler SA, Bell GI, Seino S, Le Beau MM, Westbrook CA, Neuman W, Shapiro LJ, Mohandas TK, Roessler BJ (1990) Cloning of cDNAs for human phosphoribosylpyrophosphate synthetases 1 and 2 and X chromosome localization of PRPS1 and PRPS2 genes. Genomics 8:555–56110.1016/0888-7543(90)90043-T - DOI - PubMed
-
- Taira M, Iizasa T, Yamada K, Shimada H, Tatibana M (1989) Tissue-differential expression of two distinct genes for phosphoribosyl pyrophosphate synthetase and existence of the testis-specific transcript. Biochim Biophys Acta 1007:203–208 - PubMed
-
- Becker MA, Losman MJ, Kim M (1987) Mechanisms of accelerated purine nucleotide synthesis in human fibroblasts with superactive phosphoribosylpyrophosphate synthetases. J Biol Chem 262:5596–5602 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

