Type and level of RMRP functional impairment predicts phenotype in the cartilage hair hypoplasia-anauxetic dysplasia spectrum
- PMID: 17701897
- PMCID: PMC1950841
- DOI: 10.1086/521034
Type and level of RMRP functional impairment predicts phenotype in the cartilage hair hypoplasia-anauxetic dysplasia spectrum
Abstract
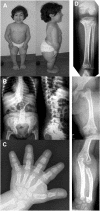
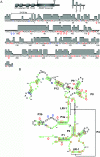
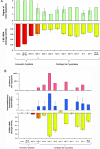
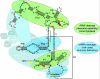
Mutations in the RMRP gene lead to a wide spectrum of autosomal recessive skeletal dysplasias, ranging from the milder phenotypes metaphyseal dysplasia without hypotrichosis and cartilage hair hypoplasia (CHH) to the severe anauxetic dysplasia (AD). This clinical spectrum includes different degrees of short stature, hair hypoplasia, defective erythrogenesis, and immunodeficiency. The RMRP gene encodes the untranslated RNA component of the mitochondrial RNA-processing ribonuclease, RNase MRP. We recently demonstrated that mutations may affect both messenger RNA (mRNA) and ribosomal RNA (rRNA) cleavage and thus cell-cycle regulation and protein synthesis. To investigate the genotype-phenotype correlation, we analyzed the position and the functional effect of 13 mutations in patients with variable features of the CHH-AD spectrum. Those at the end of the spectrum include a novel patient with anauxetic dysplasia who was compound heterozygous for the null mutation g.254_263delCTCAGCGCGG and the mutation g.195C-->T, which was previously described in patients with milder phenotypes. Mapping of nucleotide conservation to the two-dimensional structure of the RMRP gene revealed that disease-causing mutations either affect evolutionarily conserved nucleotides or are likely to alter secondary structure through mispairing in stem regions. In vitro testing of RNase MRP multiprotein-specific mRNA and rRNA cleavage of different mutations revealed a strong correlation between the decrease in rRNA cleavage in ribosomal assembly and the degree of bone dysplasia, whereas reduced mRNA cleavage, and thus cell-cycle impairment, predicts the presence of hair hypoplasia, immunodeficiency, and hematological abnormalities and thus increased cancer risk.
Figures
Similar articles
-
The molecular basis of the cartilage-hair hypoplasia-anauxetic dysplasia spectrum.Best Pract Res Clin Endocrinol Metab. 2011 Feb;25(1):131-42. doi: 10.1016/j.beem.2010.08.004. Best Pract Res Clin Endocrinol Metab. 2011. PMID: 21396580 Review.
-
Cartilage-hair hypoplasia-anauxetic dysplasia spectrum disorders harboring RMRP mutations in two Korean children: A case report.Medicine (Baltimore). 2024 May 24;103(21):e37247. doi: 10.1097/MD.0000000000037247. Medicine (Baltimore). 2024. PMID: 38787970 Free PMC article.
-
Severely incapacitating mutations in patients with extreme short stature identify RNA-processing endoribonuclease RMRP as an essential cell growth regulator.Am J Hum Genet. 2005 Nov;77(5):795-806. doi: 10.1086/497708. Epub 2005 Sep 29. Am J Hum Genet. 2005. PMID: 16252239 Free PMC article.
-
An infant with cartilage-hair hypoplasia due to a novel homozygous mutation in the promoter region of the RMRP gene associated with chondrodysplasia and severe immunodeficiency.J Appl Genet. 2010;51(4):523-8. doi: 10.1007/BF03208884. J Appl Genet. 2010. PMID: 21063072
-
RMRP-related short stature: A report of six additional Japanese individuals with cartilage hair hypoplasia and literature review.Am J Med Genet A. 2024 Jun;194(6):e63562. doi: 10.1002/ajmg.a.63562. Epub 2024 Feb 9. Am J Med Genet A. 2024. PMID: 38337186 Review.
Cited by
-
Pulmonary Follow-Up Imaging in Cartilage-Hair Hypoplasia: a Prospective Cohort Study.J Clin Immunol. 2021 Jul;41(5):1064-1071. doi: 10.1007/s10875-021-01007-5. Epub 2021 Mar 5. J Clin Immunol. 2021. PMID: 33675005 Free PMC article.
-
Functional characterization of the Drosophila MRP (mitochondrial RNA processing) RNA gene.RNA. 2010 Nov;16(11):2120-30. doi: 10.1261/rna.2227710. Epub 2010 Sep 20. RNA. 2010. PMID: 20855541 Free PMC article.
-
A 30-Year Prospective Follow-Up Study Reveals Risk Factors for Early Death in Cartilage-Hair Hypoplasia.Front Immunol. 2019 Jul 16;10:1581. doi: 10.3389/fimmu.2019.01581. eCollection 2019. Front Immunol. 2019. PMID: 31379817 Free PMC article.
-
Ribosomopathies: Global process, tissue specific defects.Rare Dis. 2015 Apr 1;3(1):e1025185. doi: 10.1080/21675511.2015.1025185. eCollection 2015. Rare Dis. 2015. PMID: 26442198 Free PMC article. Review.
-
Identification of Novel and Recurrent RMRP Variants in a Series of Brazilian Patients with Cartilage-Hair Hypoplasia: McKusick Syndrome.Mol Syndromol. 2020 Jan;10(5):255-263. doi: 10.1159/000501892. Epub 2019 Aug 15. Mol Syndromol. 2020. PMID: 32021596 Free PMC article.
References
Web Resources
-
- ClustalW, http://www.ebi.ac.uk/clustalw/
-
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for MDWH, CHH, and AD)
-
- UCSC Genome Browser, http://genome.ucsc.edu/cgi-bin/hgGateway
References
-
- Thiel CT, Horn D, Zabel B, Ekici AB, Salinas K, Gebhart E, Ruschendorf F, Sticht H, Spranger J, Muller D, et al (2005) Severely incapacitating mutations in patients with extreme short stature identify RNA-processing endoribonuclease RMRP as an essential cell growth regulator. Am J Hum Genet 77:795–806 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

