Nightly treatment of primary insomnia with eszopiclone for six months: effect on sleep, quality of life, and work limitations
- PMID: 17702264
- PMCID: PMC1978384
- DOI: 10.1093/sleep/30.8.959
Nightly treatment of primary insomnia with eszopiclone for six months: effect on sleep, quality of life, and work limitations
Abstract
Study objectives: To evaluate 6 months' eszopiclone treatment upon patient-reported sleep, fatigue and sleepiness, insomnia severity, quality of life, and work limitations.
Design: Randomized, double blind, controlled clinical trial.
Setting: 54 research sites in the U.S.
Patients: 830 primary insomnia patients who reported mean nightly total sleep time (TST) < or = 6.5 hours/night and/or mean nightly sleep latency (SL) >30 min.
Intervention: Eszopiclone 3 mg or matching placebo.
Measurements: Patient-reported sleep measures, Insomnia Severity Index, Medical Outcomes Study Short-Form Health Survey (SF-36), Work Limitations Questionnaire, and other assessments measured during baseline, treatment Months 1-6, and 2 weeks following discontinuation of treatment.
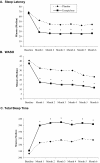
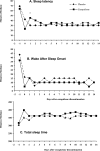
Results: Patient-reported sleep and daytime function were improved more with eszopiclone than with placebo at all months (P <0.001). Eszopiclone reduced Insomnia Severity Index scores to below clinically meaningful levels for 50% of patients (vs 19% with placebo; P <0.05) at Month 6. SF-36 domains of Physical Functioning, Vitality, and Social Functioning were improved with eszopiclone vs placebo for the Month 1-6 average (P < 0.05). Similarly, improvements were observed for all domains of the Work Limitations Questionnaire with eszopiclone vs placebo for the Month 1-6 average (P <0.05).
Conclusions: This is the first placebo-controlled investigation to demonstrate that long-term nightly pharmacologic treatment of primary insomnia with any hypnotic enhanced quality of life, reduced work limitations, and reduced global insomnia severity, in addition to improving patient-reported sleep variables.
Figures

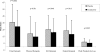
References
-
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: American Psychiatric Publishing; 1994. Sleep disorders; pp. 597–661.
-
- Morin MM. Measuring outcomes in randomized clinical trials of insomnia treatments. Sleep Med Rev. 2003;4:263–79. - PubMed
-
- Katz DA, McHorney CA. The relationship between insomnia and health-related quality of life in patients with chronic illness. J Fam Pract. 2002;51:229–35. - PubMed
-
- Leger D, Scheurmaier K, Philip P, Paillard M, Guilleminault C. SF-36: evaluation of quality of life in severe and mild insomniac compared with good sleepers. Psychosom Med. 2001;63:49–55. - PubMed
-
- National Institutes of Health. National Institutes of Health State of the Science Conference statement on Manifestations and Management of Chronic Insomnia in Adults, June 13-15, 2005. Sleep. 2005;28:1049–57. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

