Drosophila BubR1 is essential for meiotic sister-chromatid cohesion and maintenance of synaptonemal complex
- PMID: 17702574
- PMCID: PMC5629868
- DOI: 10.1016/j.cub.2007.07.042
Drosophila BubR1 is essential for meiotic sister-chromatid cohesion and maintenance of synaptonemal complex
Abstract
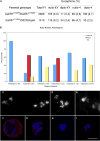
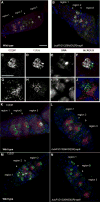
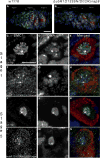
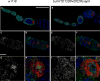
The partially conserved Mad3/BubR1 protein is required during mitosis for the spindle assembly checkpoint (SAC). In meiosis, depletion causes an accelerated transit through prophase I and missegregation of achiasmate chromosomes in yeast [1], whereas in mice, reduced dosage leads to severe chromosome missegregation [2]. These observations indicate a meiotic requirement for BubR1, but its mechanism of action remains unknown. We identified a viable bubR1 allele in Drosophila resulting from a point mutation in the kinase domain that retains mitotic SAC activity. In males, we demonstrate a dose-sensitive requirement for BubR1 in maintaining sister-chromatid cohesion at anaphase I, whereas the mutant BubR1 protein localizes correctly. In bubR1 mutant females, we find that both achiasmate and chiasmate chromosomes nondisjoin mostly equationally consistent with a defect in sister-chromatid cohesion at late anaphase I or meiosis II. Moreover, mutations in bubR1 cause a consistent increase in pericentric heterochromatin exchange frequency, and although the synaptonemal complex is set up properly during transit through the germarium, it is disassembled prematurely in prophase by stage 1. Our results demonstrate that BubR1 is essential to maintain sister-chromatid cohesion during meiotic progression in both sexes and for normal maintenance of SC in females.
Figures
Similar articles
-
Heterochromatin-mediated association of achiasmate homologs declines with age when cohesion is compromised.Genetics. 2009 Apr;181(4):1207-18. doi: 10.1534/genetics.108.099846. Epub 2009 Feb 9. Genetics. 2009. PMID: 19204374 Free PMC article.
-
Maintenance of sister-chromatid cohesion at the centromere by the Drosophila MEI-S332 protein.Genes Dev. 1998 Dec 15;12(24):3843-56. doi: 10.1101/gad.12.24.3843. Genes Dev. 1998. PMID: 9869638 Free PMC article.
-
Sisters unbound is required for meiotic centromeric cohesion in Drosophila melanogaster.Genetics. 2014 Nov;198(3):947-65. doi: 10.1534/genetics.114.166009. Epub 2014 Sep 5. Genetics. 2014. PMID: 25194162 Free PMC article.
-
The molecular basis of sister-chromatid cohesion.Annu Rev Cell Dev Biol. 2001;17:753-77. doi: 10.1146/annurev.cellbio.17.1.753. Annu Rev Cell Dev Biol. 2001. PMID: 11687503 Review.
-
Sister chromatid cohesiveness: vital function, obscure mechanism.Biochem Cell Biol. 1990 Nov;68(11):1231-42. doi: 10.1139/o90-183. Biochem Cell Biol. 1990. PMID: 2275802 Review.
Cited by
-
Regulation of APC/C activators in mitosis and meiosis.Annu Rev Cell Dev Biol. 2008;24:475-99. doi: 10.1146/annurev.cellbio.041408.115949. Annu Rev Cell Dev Biol. 2008. PMID: 18598214 Free PMC article. Review.
-
Sister centromere fusion during meiosis I depends on maintaining cohesins and destabilizing microtubule attachments.PLoS Genet. 2019 May 31;15(5):e1008072. doi: 10.1371/journal.pgen.1008072. eCollection 2019 May. PLoS Genet. 2019. PMID: 31150390 Free PMC article.
-
Interplay between synaptonemal complex, homologous recombination, and centromeres during mammalian meiosis.PLoS Genet. 2012 Jun;8(6):e1002790. doi: 10.1371/journal.pgen.1002790. Epub 2012 Jun 28. PLoS Genet. 2012. PMID: 22761591 Free PMC article.
-
A spindle assembly checkpoint protein functions in prophase I arrest and prometaphase progression.Science. 2009 Nov 13;326(5955):991-4. doi: 10.1126/science.1175326. Science. 2009. PMID: 19965510 Free PMC article.
-
A knockout screen for protein kinases required for the proper meiotic segregation of chromosomes in the fission yeast Schizosaccharomyces pombe.Cell Cycle. 2013 Feb 15;12(4):618-24. doi: 10.4161/cc.23513. Epub 2013 Jan 31. Cell Cycle. 2013. PMID: 23370392 Free PMC article.
References
-
- Cheslock PS, Kemp BJ, Boumil RM, Dawson DS. The roles of MAD1, MAD2 and MAD3 in meiotic progression and the segregation of nonexchange chromosomes. Nat. Genet. 2005;37:756–760. - PubMed
-
- Baker DJ, Jeganathan KB, Cameron JD, Thompson M, Juneja S, Kopecka A, Kumar R, Jenkins RB, de Groen PC, Roche P, van Deursen JM. BubR1 insufficiency causes early onset of aging-associated phenotypes and infertility in mice. Nat. Genet. 2004;36:744–749. - PubMed
-
- Manheim EA, McKim KS. The Synaptonemal complex component C(2)M regulates meiotic crossing over in Drosophila. Curr. Biol. 2003;13:276–285. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

