Membrane type 1-matrix metalloproteinase: substrate diversity in pericellular proteolysis
- PMID: 17702616
- PMCID: PMC2685078
- DOI: 10.1016/j.semcdb.2007.06.008
Membrane type 1-matrix metalloproteinase: substrate diversity in pericellular proteolysis
Abstract
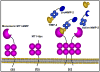
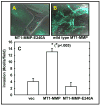
Enzymes in the matrix metalloproteinase (MMP) family have been linked to key events in developmental biology for almost 50 years. Biochemical, cellular and in vivo analyses have established that pericellular proteolysis contributes to numerous aspects of ontogeny including ovulation, fertilization, implantation, cellular migration, tissue remodeling and repair. Surface anchoring of proteinase activity provides spatial restrictions on substrate targeting. This review will utilize membrane type 1 MMP (MT1-MMP) as an example to highlight substrate diversity in pericellular proteolysis catalyzed by a membrane anchored MMP.
Figures


Similar articles
-
MT1-MMP-dependent cell migration: proteolytic and non-proteolytic mechanisms.Biochem Soc Trans. 2019 Jun 28;47(3):811-826. doi: 10.1042/BST20180363. Epub 2019 May 7. Biochem Soc Trans. 2019. PMID: 31064864 Free PMC article. Review.
-
Matrix metalloproteinase-14 both sheds cell surface neuronal glial antigen 2 (NG2) proteoglycan on macrophages and governs the response to peripheral nerve injury.J Biol Chem. 2015 Feb 6;290(6):3693-707. doi: 10.1074/jbc.M114.603431. Epub 2014 Dec 8. J Biol Chem. 2015. PMID: 25488667 Free PMC article.
-
Identification and characterization of Lutheran blood group glycoprotein as a new substrate of membrane-type 1 matrix metalloproteinase 1 (MT1-MMP): a systemic whole cell analysis of MT1-MMP-associating proteins in A431 cells.J Biol Chem. 2009 Oct 2;284(40):27360-9. doi: 10.1074/jbc.M109.029124. Epub 2009 Aug 10. J Biol Chem. 2009. PMID: 19667067 Free PMC article.
-
Tetraspanin proteins regulate membrane type-1 matrix metalloproteinase-dependent pericellular proteolysis.Mol Biol Cell. 2009 Apr;20(7):2030-40. doi: 10.1091/mbc.e08-11-1149. Epub 2009 Feb 11. Mol Biol Cell. 2009. PMID: 19211836 Free PMC article.
-
MT1-MMP: a key regulator of cell migration in tissue.IUBMB Life. 2006 Oct;58(10):589-96. doi: 10.1080/15216540600962818. IUBMB Life. 2006. PMID: 17050376 Review.
Cited by
-
Non-destructive and selective imaging of the functionally active, pro-invasive membrane type-1 matrix metalloproteinase (MT1-MMP) enzyme in cancer cells.J Biol Chem. 2013 Jul 12;288(28):20568-80. doi: 10.1074/jbc.M113.471508. Epub 2013 Jun 3. J Biol Chem. 2013. PMID: 23733191 Free PMC article.
-
Proteolytic cleavage of membrane proteins by membrane type-1 MMP regulates cancer malignant progression.Cancer Sci. 2023 Feb;114(2):348-356. doi: 10.1111/cas.15638. Epub 2022 Nov 24. Cancer Sci. 2023. PMID: 36336966 Free PMC article. Review.
-
Structural basis for the sheddase function of human meprin β metalloproteinase at the plasma membrane.Proc Natl Acad Sci U S A. 2012 Oct 2;109(40):16131-6. doi: 10.1073/pnas.1211076109. Epub 2012 Sep 17. Proc Natl Acad Sci U S A. 2012. PMID: 22988105 Free PMC article.
-
A membrane-type-1 matrix metalloproteinase (MT1-MMP)-discoidin domain receptor 1 axis regulates collagen-induced apoptosis in breast cancer cells.PLoS One. 2015 Mar 16;10(3):e0116006. doi: 10.1371/journal.pone.0116006. eCollection 2015. PLoS One. 2015. PMID: 25774665 Free PMC article.
-
Physiology and pathophysiology of matrix metalloproteases.Amino Acids. 2011 Jul;41(2):271-90. doi: 10.1007/s00726-010-0689-x. Epub 2010 Jul 18. Amino Acids. 2011. PMID: 20640864 Free PMC article. Review.
References
-
- Ellerbroek SM, Stack MS. Membrane associated matrix metalloproteinases in metastasis. Bioessays. 1999;21(11):940–949. - PubMed
-
- Ellerbroek SM, Stack MS. Regulatory Mechanisms for Proteinase Activity. In: Simons HJSaC., editor. Proteinase and Peptidase Inhibition. Taylor and Francis; 2002. pp. 21–34.
-
- Werb Z. ECM and cell surface proteolysis: regulating cellular ecology. Cell. 1997;91(4):439–442. - PubMed
-
- Sato H, Takino T, Kinoshita T, et al. Cell surface binding and activation of gelatinase A induced by expression of membrane-type-1-matrix metalloproteinase (MT1-MMP) FEBS Letters. 1996;385(3):238–240. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

