Role of modern chemistry in sustainable arable crop protection
- PMID: 17702697
- PMCID: PMC2610174
- DOI: 10.1098/rstb.2007.2174
Role of modern chemistry in sustainable arable crop protection
Abstract
Organic chemistry has been, and for the foreseeable future will remain, vitally important for crop protection. Control of fungal pathogens, insect pests and weeds is crucial to enhanced food provision. As world population continues to grow, it is timely to assess the current situation, anticipate future challenges and consider how new chemistry may help meet those challenges. In future, agriculture will increasingly be expected to provide not only food and feed, but also crops for conversion into renewable fuels and chemical feedstocks. This will further increase the demand for higher crop yields per unit area, requiring chemicals used in crop production to be even more sophisticated. In order to contribute to programmes of integrated crop management, there is a requirement for chemicals to display high specificity, demonstrate benign environmental and toxicological profiles, and be biodegradable. It will also be necessary to improve production of those chemicals, because waste generated by the production process mitigates the overall benefit. Three aspects are considered in this review: advances in the discovery process for new molecules for sustainable crop protection, including tests for environmental and toxicological properties as well as biological activity; advances in synthetic chemistry that may offer efficient and environmentally benign manufacturing processes for modern crop protection chemicals; and issues related to energy use and production through agriculture.
Figures
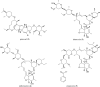
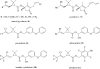

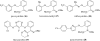
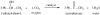
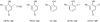
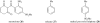
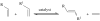
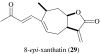
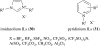
References
-
- Anastas, P. T. 1994 Benign by design chemistry. In Benign by design: alternative synthetic design for pollution prevention (eds P. T. Anastas & C. A. Farris). ACS Symposium Series, no. 577, pp. 1–21. Washington, DC: American Chemical Society.
-
- Anastas P.T, Warner J.C. Oxford University Press; New York, NY: 1998. Green chemistry: theory and practice.
-
- Ashford R.D. Wavelength Publication; London, UK: 1994. Ashford's dictionary of industrial chemicals.
-
- Atkin J, Leisinger K.M, editors. Safe and effective use of crop protection chemicals in developing countries. CABI Publishing; Wallingford, UK: 2000. pp. 1–14.
-
- Bartlett D.W, Clough J.M, Godwin J.R, Hall A.A, Hamer M, Parr-Dobrzanski B. The strobilurin fungicides. Pest Manage. Sci. 2002;58:649–662. doi:10.1002/ps.520 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
