Establishment of transplantable porcine tumor cell lines derived from MHC-inbred miniature swine
- PMID: 17702898
- PMCID: PMC2190613
- DOI: 10.1182/blood-2007-02-074450
Establishment of transplantable porcine tumor cell lines derived from MHC-inbred miniature swine
Abstract
The lack of transplantable tumors has limited assessment of graft-versus-tumor effects following hematopoietic cell transplantation in clinically relevant large-animal models. We describe the derivation and characterization of porcine tumor cell lines with initial efforts of tumor transplantation using immunocompromised mice and highly inbred sublines of Massachusetts General Hospital major histocompatibility complex (MHC)-inbred miniature swine. Autopsies were performed routinely on swine that died unexpectedly or had suspicion of malignancy based on clinical symptoms or peripheral blood analysis. Tissue samples were obtained for pathology, phenotyped by flow cytometry, and placed in culture. Based on growth, lines were selected for passage into nonobese diabetic/severe combined immunodeficient (NOD/SCID) mice and miniature swine. Porcine tumor recipients were preconditioned with total body irradiation from 0 to 500 cGy or with a 30-day course of oral cyclosporine. We identified 19 cases of hematologic tumors. Nine distinct tumor cell lines were established from 8 of these cases, including 3 derived from highly inbred sublines. In vivo tumor growth and serial transfer were observed in immunocompromised mice for one tumor cell line and in miniature swine for 1 of 2 tumor cell lines expanded for this purpose. These results suggest the possibility of developing a transplantable tumor model in this large-animal system.
Figures
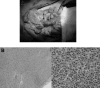
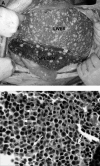



References
-
- Adelman CA, Petrini JH, Attwooll CL. Modeling disease in the mouse: lessons from DNA damage response and cell cycle control genes. J Cell Biochem. 2006;97:459–473. - PubMed
-
- Braithwaite AW, Royds JA, Jackson P. The p53 story: layers of complexity. Carcinogenesis. 2005;26:1161–1169. - PubMed
-
- Decker S, Sausville EA. Preclinical modeling of combination treatments: fantasy or requirement? Ann N Y Acad Sci. 2005;1059:61–69. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

