Visco-elastic membrane tethers extracted from Escherichia coli by optical tweezers
- PMID: 17704145
- PMCID: PMC2084229
- DOI: 10.1529/biophysj.107.103861
Visco-elastic membrane tethers extracted from Escherichia coli by optical tweezers
Abstract
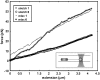
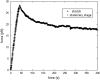
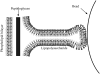
Tethers were created between a living Escherichia coli bacterium and a bead by unspecifically attaching the bead to the outer membrane and pulling it away using optical tweezers. Upon release, the bead returned to the bacterium, thus showing the existence of an elastic tether between the bead and the bacterium. These tethers can be tens of microns long, several times the bacterial length. Using mutants expressing different parts of the outer membrane structure, we have shown that an intact core lipopolysaccharide is a necessary condition for tether formation, regardless of whether the beads were uncoated polystyrene or beads coated with lectin. A physical characterization of the tethers has been performed yielding visco-elastic tether force-extension relationships: for first pull tethers, a spring constant of 10-12 pN/mum describes the tether visco-elasticity, for subsequent pulls the spring constant decreases to 6-7 pN/mum, and typical relaxation timescales of hundreds of seconds are observed. Studies of tether stability in the presence of proteases, lipases, and amylases lead us to propose that the extracted tether is primarily composed of the asymmetric lipopolysaccharide containing bilayer of the outer membrane. This unspecific tethered attachment mechanism could be important in the initiation of bacterial adhesion.
Figures



Similar articles
-
Membrane tether formation from outer hair cells with optical tweezers.Biophys J. 2002 Mar;82(3):1386-95. doi: 10.1016/S0006-3495(02)75493-3. Biophys J. 2002. PMID: 11867454 Free PMC article.
-
Cell protrusions and tethers: a unified approach.Biophys J. 2011 Apr 6;100(7):1697-707. doi: 10.1016/j.bpj.2011.02.038. Biophys J. 2011. PMID: 21463583 Free PMC article.
-
The motion of a single molecule, the lambda-receptor, in the bacterial outer membrane.Biophys J. 2002 Dec;83(6):3152-61. doi: 10.1016/S0006-3495(02)75318-6. Biophys J. 2002. PMID: 12496085 Free PMC article.
-
Cell membrane biophysics with optical tweezers.Eur Biophys J. 2018 Jul;47(5):499-514. doi: 10.1007/s00249-017-1268-9. Epub 2017 Nov 21. Eur Biophys J. 2018. PMID: 29164289 Review.
-
Physical properties of biopolymers assessed by optical tweezers: analysis of folding and refolding of bacterial pili.Chemphyschem. 2008 Feb 1;9(2):221-35. doi: 10.1002/cphc.200700389. Chemphyschem. 2008. PMID: 18181116 Review.
Cited by
-
Membrane tubulovesicular extensions (cytonemes): secretory and adhesive cellular organelles.Cell Adh Migr. 2013 Mar-Apr;7(2):174-86. doi: 10.4161/cam.23130. Epub 2013 Jan 3. Cell Adh Migr. 2013. PMID: 23287580 Free PMC article. Review.
-
Extensible membrane nanotubules mediate attachment of Trypanosoma cruzi epimastigotes under flow.PLoS One. 2023 Mar 22;18(3):e0283182. doi: 10.1371/journal.pone.0283182. eCollection 2023. PLoS One. 2023. PMID: 36947570 Free PMC article.
-
Experimental study of the difference in deformation between normal and pathological, renal and bladder, cells induced by acoustic radiation force.Eur Biophys J. 2020 Mar;49(2):155-161. doi: 10.1007/s00249-020-01422-3. Epub 2020 Jan 31. Eur Biophys J. 2020. PMID: 32006056
-
Altered Envelope Structure and Nanomechanical Properties of a C-Terminal Protease A-Deficient Rhizobium leguminosarum.Microorganisms. 2020 Sep 16;8(9):1421. doi: 10.3390/microorganisms8091421. Microorganisms. 2020. PMID: 32947797 Free PMC article.
-
Effect of energy metabolism on protein motility in the bacterial outer membrane.Biophys J. 2009 Sep 2;97(5):1305-12. doi: 10.1016/j.bpj.2009.06.027. Biophys J. 2009. PMID: 19720018 Free PMC article.
References
-
- Koster, G., A. Cacciuto, I. Dereyi, D. Frankel, and M. Dogterom. 2005. Force barriers for membrane tube formation. Phys. Rev. Lett. 94:068101. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

