Biophysical characterization of anticoagulant hemextin AB complex from the venom of snake Hemachatus haemachatus
- PMID: 17704148
- PMCID: PMC2084224
- DOI: 10.1529/biophysj.106.100164
Biophysical characterization of anticoagulant hemextin AB complex from the venom of snake Hemachatus haemachatus
Abstract
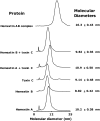
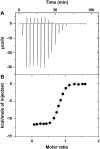
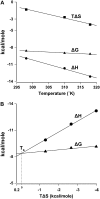
Hemextin AB complex from the venom of Hemachatus haemachatus is the first known natural anticoagulant that specifically inhibits the enzymatic activity of blood coagulation factor VIIa in the absence of factor Xa. It is also the only known heterotetrameric complex of two three-finger toxins. Individually only hemextin A has mild anticoagulant activity, whereas hemextin B is inactive. However, hemextin B synergistically enhances the anticoagulant activity of hemextin A and their complex exhibits potent anticoagulant activity. In this study we characterized the nature of molecular interactions leading to the complex formation. Circular dichroism studies indicate the stabilization of beta-sheet in the complex. Hemextin AB complex has an increased apparent molecular diameter in both gas and liquid phase techniques. The complex formation is enthalpically favorable and entropically unfavorable with a negative change in the heat capacity. Thus, the anticoagulant complex shows less structural flexibility than individual subunits. Both electrostatic and hydrophobic interactions are important for the complexation; the former driving the process and the latter helping in the stabilization of the tetramer. The tetramer dissociates into dimers and monomers with the increase in the ionic strength of the solution and also with increase in the glycerol concentration in the buffer. The two dimers formed under each of these conditions display distinct differences in their apparent molecular diameters and anticoagulant properties. Based on these results, we have proposed a model for this unique anticoagulant complex.
Figures




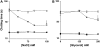

Similar articles
-
Hemextin AB complex--a snake venom anticoagulant protein complex that inhibits factor VIIa activity.Pathophysiol Haemost Thromb. 2005;34(4-5):184-7. doi: 10.1159/000092420. Pathophysiol Haemost Thromb. 2005. PMID: 16707924
-
Hemextin AB complex, a unique anticoagulant protein complex from Hemachatus haemachatus (African Ringhals cobra) venom that inhibits clot initiation and factor VIIa activity.J Biol Chem. 2005 Dec 30;280(52):42601-11. doi: 10.1074/jbc.M508987200. Epub 2005 Oct 4. J Biol Chem. 2005. PMID: 16204244
-
Crystallization and preliminary X-ray diffraction analysis of hemextin A: a unique anticoagulant protein from Hemachatus haemachatus venom.Acta Crystallogr Sect F Struct Biol Cryst Commun. 2007 Aug 1;63(Pt 8):701-3. doi: 10.1107/S1744309107034239. Epub 2007 Jul 21. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2007. PMID: 17671372 Free PMC article.
-
Structure-function relationships and mechanism of anticoagulant phospholipase A2 enzymes from snake venoms.Toxicon. 2005 Jun 15;45(8):1147-61. doi: 10.1016/j.toxicon.2005.02.018. Epub 2005 Apr 13. Toxicon. 2005. PMID: 15922780 Review.
-
Structure and function of snake venom toxins interacting with human von Willebrand factor.Toxicon. 2005 Jun 15;45(8):1075-87. doi: 10.1016/j.toxicon.2005.02.023. Epub 2005 Apr 22. Toxicon. 2005. PMID: 15922776 Review.
Cited by
-
In Silico Design of Novel Anticoagulant Peptides targeting Blood Coagulation Factor VIIa.Sultan Qaboos Univ Med J. 2011 Feb;11(1):83-94. Epub 2011 Feb 12. Sultan Qaboos Univ Med J. 2011. PMID: 21509213 Free PMC article.
-
The Search for Natural and Synthetic Inhibitors That Would Complement Antivenoms as Therapeutics for Snakebite Envenoming.Toxins (Basel). 2021 Jun 29;13(7):451. doi: 10.3390/toxins13070451. Toxins (Basel). 2021. PMID: 34209691 Free PMC article. Review.
-
The chemistry of snake venom and its medicinal potential.Nat Rev Chem. 2022;6(7):451-469. doi: 10.1038/s41570-022-00393-7. Epub 2022 Jun 10. Nat Rev Chem. 2022. PMID: 35702592 Free PMC article. Review.
-
Quantitative Characterization of the Hemorrhagic, Necrotic, Coagulation-Altering Properties and Edema-Forming Effects of Zebra Snake (Naja nigricincta nigricincta) Venom.J Toxicol. 2018 Oct 24;2018:6940798. doi: 10.1155/2018/6940798. eCollection 2018. J Toxicol. 2018. PMID: 30473709 Free PMC article.
-
A Venomics Approach to the Identification and Characterization of Bioactive Peptides From Animal Venoms for Colorectal Cancer Therapy: Protocol for a Proof-of-Concept Study.JMIR Res Protoc. 2021 Dec 21;10(12):e31128. doi: 10.2196/31128. JMIR Res Protoc. 2021. PMID: 34932002 Free PMC article.
References
-
- Davie, E. W., K. Fujikawa, and W. Kisiel. 1991. The coagulation cascade: initiation, maintenance, and regulation. Biochemistry. 30:10363–10370. - PubMed
-
- Davie, E. W. 1995. Biochemical and molecular aspects of the coagulation cascade. Thromb. Haemost. 74:1–6. - PubMed
-
- Nemerson, Y. 1988. Tissue factor and hemostasis. Blood. 71:1–8. - PubMed
-
- Yegneswaran, S., R. M. Mesters, and J. H. Griffin. 2003. Identification of distinct sequences in human blood coagulation factor Xa and prothrombin essential for substrate and cofactor recognition in the prothrombinase complex. J. Biol. Chem. 278:33312–33318. - PubMed
-
- Kumar, R., S. Beguin, and H. C. Hemker. 1995. The effect of fibrin clots and clot-bound thrombin on the development of platelet procoagulant activity. Thromb. Haemost. 74:962–968. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

