Simultaneous tether extraction from endothelial cells and leukocytes: observation, mechanics, and significance
- PMID: 17704170
- PMCID: PMC2084253
- DOI: 10.1529/biophysj.107.109298
Simultaneous tether extraction from endothelial cells and leukocytes: observation, mechanics, and significance
Abstract
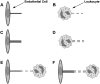
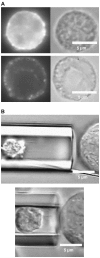
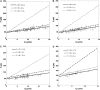
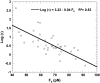
It has been hypothesized, from earlier studies on single-tether extraction from individual leukocytes and human umbilical vein endothelial cells, that during rolling of leukocytes on the endothelium, simultaneous extraction of membrane nanotubes (tethers) occurs, resulting in enhancement of the force decrease on the adhesive bond. In this study, using the micropipette aspiration technique and fluorescence microscopy, we show that tethers are indeed extracted simultaneously when an endothelial cell and a leukocyte are separated after brief contact and adhesion, and the endothelial cell contributes much more to the composite tether length. In addition, the constitutive relationship for simultaneous tether extraction is determined with neutrophils and T-lymphocytes as force transducers, and cytokine-stimulated human umbilical vein and dermal microvascular endothelial cells as substrates, respectively. This relationship is consistent with that derived theoretically from the constitutive equations for single-tether extraction from either cell alone. Moreover, we show that simultaneous tether extraction was likely terminated by receptor-ligand bond dissociation. With a biomechanical model of leukocyte rolling, we predict the force history of the adhesive receptor-ligand bond and show that it is remarkably similar for different leukocyte-endothelial cell pairs. Simultaneous tether extraction therefore represents a generic mechanism for stabilizing leukocyte rolling on the endothelium.
Figures


Similar articles
-
Double-tether extraction from human umbilical vein and dermal microvascular endothelial cells.Biophys J. 2007 Feb 1;92(3):1035-45. doi: 10.1529/biophysj.106.086256. Epub 2006 Nov 10. Biophys J. 2007. PMID: 17098792 Free PMC article.
-
Membrane tether extraction from human umbilical vein endothelial cells and its implication in leukocyte rolling.Biophys J. 2004 Nov;87(5):3561-8. doi: 10.1529/biophysj.104.047514. Epub 2004 Aug 31. Biophys J. 2004. PMID: 15339799 Free PMC article.
-
Simultaneous tether extraction contributes to neutrophil rolling stabilization: a model study.Biophys J. 2007 Jan 15;92(2):418-29. doi: 10.1529/biophysj.105.078808. Epub 2006 Oct 27. Biophys J. 2007. PMID: 17071668 Free PMC article.
-
Trans-cellular migration: cell-cell contacts get intimate.Curr Opin Cell Biol. 2008 Oct;20(5):533-40. doi: 10.1016/j.ceb.2008.05.007. Epub 2008 Jul 1. Curr Opin Cell Biol. 2008. PMID: 18595683 Free PMC article. Review.
-
Endothelial cell interactions and integrins.New Horiz. 1993 Feb;1(1):37-51. New Horiz. 1993. PMID: 7922392 Review.
Cited by
-
Stiffness based enrichment of leukemia cells using microfluidics.APL Bioeng. 2020 Jul 1;4(3):036101. doi: 10.1063/1.5143436. eCollection 2020 Sep. APL Bioeng. 2020. PMID: 32637856 Free PMC article.
-
Cytoskeleton modification and cholesterol depletion affect membrane properties and caveolae positioning of CHO cells.J Membr Biol. 2014 Mar;247(3):201-10. doi: 10.1007/s00232-013-9625-9. Epub 2014 Jan 11. J Membr Biol. 2014. PMID: 24413749
-
Biomechanics of Neutrophil Tethers.Life (Basel). 2021 May 31;11(6):515. doi: 10.3390/life11060515. Life (Basel). 2021. PMID: 34073130 Free PMC article. Review.
-
Combined atomic force microscopy and side-view optical imaging for mechanical studies of cells.Nat Methods. 2009 May;6(5):383-7. doi: 10.1038/nmeth.1320. Epub 2009 Apr 12. Nat Methods. 2009. PMID: 19363493 Free PMC article.
-
Validation, In-Depth Analysis, and Modification of the Micropipette Aspiration Technique.Cell Mol Bioeng. 2009;2(3):351-365. doi: 10.1007/s12195-009-0071-9. Cell Mol Bioeng. 2009. PMID: 20333318 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

