Golgi regeneration after brefeldin A treatment in BY-2 cells entails stack enlargement and cisternal growth followed by division
- PMID: 17704232
- PMCID: PMC2048719
- DOI: 10.1104/pp.107.104919
Golgi regeneration after brefeldin A treatment in BY-2 cells entails stack enlargement and cisternal growth followed by division
Abstract
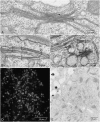
Brefeldin A (BFA) treatment stops secretion and leads to the resorption of much of the Golgi apparatus into the endoplasmic reticulum. This effect is reversible upon washing out the drug, providing a situation for studying Golgi biogenesis. In this investigation Golgi regeneration in synchronized tobacco BY-2 cells was followed by electron microscopy and by the immunofluorescence detection of ARF1, which localizes to the rims of Golgi cisternae and serves as an indicator of COPI vesiculation. Beginning as clusters of vesicles that are COPI positive, mini-Golgi stacks first become recognizable 60 min after BFA washout. They continue to increase in terms of numbers and length of cisternae for a further 90 min before overshooting the size of control Golgi stacks. As a result, increasing numbers of dividing Golgi stacks were observed 120 min after BFA washout. BFA-regeneration experiments performed on cells treated with BFA (10 microg mL(-1)) for only short periods (30-45 min) showed that the formation of ER-Golgi hybrid structures, once initiated by BFA treatment, is an irreversible process, the further incorporation of Golgi membranes into the ER continuing during a subsequent drug washout. Application of the protein kinase A inhibitor H-89, which effectively blocks the reassembly of the Golgi apparatus in mammalian cells, also prevented stack regeneration in BY-2 cells, but only at very high, almost toxic concentrations (>200 microm). Our data suggest that under normal conditions mitosis-related Golgi stack duplication may likely occur via cisternal growth followed by fission.
Figures





References
-
- Altan-Bonnet N, Sougrat R, Lippincott-Schwartz J (2004) Molecular basis for Golgi maintenance and biogenesis. Curr Opin Cell Biol 16 364–372 - PubMed
-
- Aniento F, Matsuoka K, Robinson DG (2006) ER-to-Golgi transport: the COPII-pathway. In DG Robinson, ed, The Plant Endoplasmic Reticulum. Springer-Verlag, Berlin, pp 99–124
-
- Aridor M, Balch WE (2000) Kinase signaling initiates coat complex II (COPII) recruitment and export from the mammalian endoplasmic reticulum. J Biol Chem 275 35673–35676 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

