microPET of tumor integrin alphavbeta3 expression using 18F-labeled PEGylated tetrameric RGD peptide (18F-FPRGD4)
- PMID: 17704249
- PMCID: PMC4183663
- DOI: 10.2967/jnumed.107.040816
microPET of tumor integrin alphavbeta3 expression using 18F-labeled PEGylated tetrameric RGD peptide (18F-FPRGD4)
Abstract
In vivo imaging of alpha(v)beta(3) expression has important diagnostic and therapeutic applications. Multimeric cyclic RGD peptides are capable of improving the integrin alpha(v)beta(3)-binding affinity due to the polyvalency effect. Here we report an example of (18)F-labeled tetrameric RGD peptide for PET of alpha(v)beta(3) expression in both xenograft and spontaneous tumor models.
Methods: The tetrameric RGD peptide E{E[c(RGDyK)](2)}(2) was derived with amino-3,6,9-trioxaundecanoic acid (mini-PEG; PEG is poly(ethylene glycol)) linker through the glutamate alpha-amino group. NH(2)-mini-PEG-E{E[c(RGDyK)](2)}(2) (PRGD4) was labeled with (18)F via the N-succinimidyl-4-(18)F-fluorobenzoate ((18)F-SFB) prosthetic group. The receptor-binding characteristics of the tetrameric RGD peptide tracer (18)F-FPRGD4 were evaluated in vitro by a cell-binding assay and in vivo by quantitative microPET imaging studies.
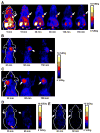
Results: The decay-corrected radiochemical yield for (18)F-FPRGD4 was about 15%, with a total reaction time of 180 min starting from (18)F-F(-). The PEGylation had minimal effect on integrin-binding affinity of the RGD peptide. (18)F-FPRGD4 has significantly higher tumor uptake compared with monomeric and dimeric RGD peptide tracer analogs. The receptor specificity of (18)F-FPRGD4 in vivo was confirmed by effective blocking of the uptake in both tumors and normal organs or tissues with excess c(RGDyK).
Conclusion: The tetrameric RGD peptide tracer (18)F-FPRGD4 possessing high integrin-binding affinity and favorable biokinetics is a promising tracer for PET of integrin alpha(v)beta(3) expression in cancer and other angiogenesis related diseases.
Figures




References
-
- Castel S, Pagan R, Mitjans F, et al. RGD peptides and monoclonal antibodies, antagonists of αv-integrin, enter the cells by independent endocytic pathways. Lab Invest. 2001;81:1615–1626. - PubMed
-
- Cheng YF, Kramer RH. Human microvascular endothelial cells express integrin-related complexes that mediate adhesion to the extracellular matrix. J Cell Physiol. 1989;139:275–286. - PubMed
-
- Hwang R, Varner J. The role of integrins in tumor angiogenesis. Hematol Oncol Clin North Am. 2004;18:991–1006. - PubMed
-
- Albelda SM, Mette SA, Elder DE, et al. Integrin distribution in malignant melanoma: association of the β3 subunit with tumor progression. Cancer Res. 1990;50:6757–6764. - PubMed
-
- Bello L, Francolini M, Marthyn P, et al. αvβ3 and αvβ5 integrin expression in glioma periphery. Neurosurgery. 2001;49:380–389. discussion 390. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
