Changes in Listeria monocytogenes membrane fluidity in response to temperature stress
- PMID: 17704268
- PMCID: PMC2075051
- DOI: 10.1128/AEM.00980-07
Changes in Listeria monocytogenes membrane fluidity in response to temperature stress
Abstract
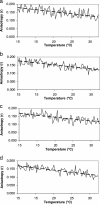
Listeria monocytogenes is a food-borne pathogen that has been implicated in many outbreaks associated with ready-to-eat products. Listeria adjusts to various stresses by adjusting its membrane fluidity, increasing the uptake of osmoprotectants and cryoprotectants, and activating the sigma(B) stress factor. The present work examines the regulation of membrane fluidity through direct measurement based on fluorescent anisotropy. The membrane fluidities of L. monocytogenes Scott A, NR30, wt10403S, and cld1 cells cultured at 15 and 30 degrees C were measured at 15 and 30 degrees C. The membrane of the cold-sensitive mutant (cld1) was more rigid than the membranes of the other strains when grown at 30 degrees C, but when grown at 15 degrees C, it was able to adjust its membrane to approach the rigidity of the other strains. The difference in rigidities, as determined at 15 and 30 degrees C, was greater in liposomes than in whole cells. The rates of fluidity adjustment and times required for whole cells to adjust to a different temperature were similar among strains but different from those of liposomes. This suggests that the cells had a mechanism for homeoviscous adaptation that was absent in liposomes.
Figures





References
-
- Bayles, D. O. 2004. Changes in heat resistance resulting from pH and nutritional shifts of acid-adapted and non-acid-adapted Listeria monocytogenes Scott A. J. Food Prot. 67:316-321. - PubMed
-
- Bligh, E. G., and W. J. Dyer. 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37:911-917. - PubMed
-
- Bolton, L. F., and J. F. Frank. 1999. Simple method to observe the adaptive response of Listeria monocytogenes in food. Lett. Appl. Microbiol. 29:350-353. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

