Role and regulation of acylethanolamides in energy balance: focus on adipocytes and beta-cells
- PMID: 17704823
- PMCID: PMC2190005
- DOI: 10.1038/sj.bjp.0707424
Role and regulation of acylethanolamides in energy balance: focus on adipocytes and beta-cells
Abstract
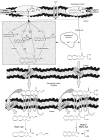
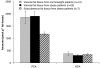
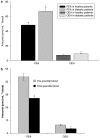
The endocannabinoid, arachidonoylethanolamide (AEA), and the peroxisome proliferator-activated receptor (PPAR)-alpha ligand, oleylethanolamide (OEA) produce opposite effects on lipogenesis. The regulation of OEA and its anti-inflammatory congener, palmitoylethanolamide (PEA), in adipocytes and pancreatic beta-cells has not been investigated. We report here the results of studies on acylethanolamide regulation in these cells during obesity and hyperglycaemia, and provide an overview of acylethanolamide role in metabolic control. We analysed by liquid chromatography-mass spectrometry OEA and PEA levels in: 1) mouse 3T3F442A adipocytes during insulin-induced differentiation, 2) rat insulinoma RIN m5F beta-cells kept in 'low' or 'high' glucose, 3) adipose tissue and pancreas of mice with high fat diet-induced obesity (DIO), and 4) in visceral fat or blood of obese or type 2 diabetes (T2D) patients. In adipocytes, OEA levels remain unchanged during differentiation, whereas those of PEA decrease significantly, and are under the negative control of both leptin and PPAR-gamma. PEA is significantly downregulated in subcutaneous adipose tissue of DIO mice. In RIN m5F insulinoma beta-cells, OEA and PEA levels are inhibited by 'very high' glucose, this effect being enhanced by insulin, whereas in cells kept for 24 h in 'high' glucose, they are stimulated by both glucose and insulin. Elevated OEA and PEA levels are found in the blood of T2D patients. Reduced PEA levels in hypertrophic adipocytes might play a role in obesity-related pro-inflammatory states. In beta-cells and human blood, OEA and PEA are down- or up-regulated under conditions of transient or chronic hyperglycaemia, respectively.
Figures



Similar articles
-
Regulation, function, and dysregulation of endocannabinoids in models of adipose and beta-pancreatic cells and in obesity and hyperglycemia.J Clin Endocrinol Metab. 2006 Aug;91(8):3171-80. doi: 10.1210/jc.2005-2679. Epub 2006 May 9. J Clin Endocrinol Metab. 2006. PMID: 16684820
-
Differential alterations of the concentrations of endocannabinoids and related lipids in the subcutaneous adipose tissue of obese diabetic patients.Lipids Health Dis. 2010 Apr 28;9:43. doi: 10.1186/1476-511X-9-43. Lipids Health Dis. 2010. PMID: 20426869 Free PMC article.
-
Role of acylethanolamides in the gastrointestinal tract with special reference to food intake and energy balance.Best Pract Res Clin Endocrinol Metab. 2009 Feb;23(1):33-49. doi: 10.1016/j.beem.2008.10.003. Best Pract Res Clin Endocrinol Metab. 2009. PMID: 19285259 Review.
-
Simultaneous measurement of three N-acylethanolamides in human bio-matrices using ultra performance liquid chromatography-tandem mass spectrometry.Anal Bioanal Chem. 2010 Nov;398(5):2089-97. doi: 10.1007/s00216-010-4103-z. Epub 2010 Sep 12. Anal Bioanal Chem. 2010. PMID: 20835819
-
Palmitoylethanolamide and other anandamide congeners. Proposed role in the diseased brain.Exp Neurol. 2010 Jul;224(1):48-55. doi: 10.1016/j.expneurol.2010.03.022. Epub 2010 Mar 29. Exp Neurol. 2010. PMID: 20353771 Review.
Cited by
-
Cannabinoids and their actions.Br J Pharmacol. 2007 Nov;152(5):557-8. doi: 10.1038/sj.bjp.0707483. Epub 2007 Sep 24. Br J Pharmacol. 2007. PMID: 17891156 Free PMC article. No abstract available.
-
A Lower Olfactory Capacity Is Related to Higher Circulating Concentrations of Endocannabinoid 2-Arachidonoylglycerol and Higher Body Mass Index in Women.PLoS One. 2016 Feb 5;11(2):e0148734. doi: 10.1371/journal.pone.0148734. eCollection 2016. PLoS One. 2016. PMID: 26849214 Free PMC article. Clinical Trial.
-
The role of endocannabinoids in the regulation of gastric emptying: alterations in mice fed a high-fat diet.Br J Pharmacol. 2008 Mar;153(6):1272-80. doi: 10.1038/sj.bjp.0707682. Epub 2008 Jan 28. Br J Pharmacol. 2008. PMID: 18223666 Free PMC article.
-
FAAH deficiency promotes energy storage and enhances the motivation for food.Int J Obes (Lond). 2010 Mar;34(3):557-68. doi: 10.1038/ijo.2009.262. Epub 2009 Dec 22. Int J Obes (Lond). 2010. PMID: 20029375 Free PMC article.
-
'Entourage' effects of N-palmitoylethanolamide and N-oleoylethanolamide on vasorelaxation to anandamide occur through TRPV1 receptors.Br J Pharmacol. 2008 Nov;155(6):837-46. doi: 10.1038/bjp.2008.324. Epub 2008 Aug 11. Br J Pharmacol. 2008. PMID: 18695637 Free PMC article.
References
-
- Ahern GP. Activation of TRPV1 by the satiety factor oleoylethanolamide. J Biol Chem. 2003;278:30429–30434. - PubMed
-
- Astarita G, Rourke BC, Andersen JB, Fu J, Kim JH, Bennett AF, et al. Postprandial increase of oleoylethanolamide mobilization in small intestine of the Burmese python (Python molurus) Am J Physiol Regul Integr Comp Physiol. 2006;290:R1407–R1412. - PubMed
-
- Bachur NR, Masek K, Melmon KL, Udenfriend S. Fatty acid amides of ethanolamine in mammalian tissues. J Biol Chem. 1965;240:1019–1024. - PubMed
-
- Bensaid M, Gary-Bobo M, Esclangon A, Maffrand JP, Le Fur G, Oury-Donat F, et al. The cannabinoid CB1 receptor antagonist SR141716 increases Acrp30 mRNA expression in adipose tissue of obese fa/fa rats and in cultured adipocyte cells. Mol Pharmacol. 2003;63:908–914. - PubMed
-
- Berdyshev E, Boichot E, Corbel M, Germain N, Lagente V. Effects of cannabinoid receptor ligands on LPS-induced pulmonary inflammation in mice. Life Sci. 1998;63:PL125–PL129. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

