Phosphatidylserine containing liposomes reduce immunogenicity of recombinant human factor VIII (rFVIII) in a murine model of hemophilia A
- PMID: 17705286
- PMCID: PMC2574438
- DOI: 10.1002/jps.21102
Phosphatidylserine containing liposomes reduce immunogenicity of recombinant human factor VIII (rFVIII) in a murine model of hemophilia A
Abstract
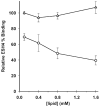
Factor VIII (FVIII) is a multidomain protein that is deficient in hemophilia A, a clinically important bleeding disorder. Replacement therapy using recombinant human FVIII (rFVIII) is the main therapy. However, approximately 15-30% of patients develop inhibitory antibodies that neutralize rFVIII activity. Antibodies to epitopes in C2 domain, which is involved in FVIII binding to phospholipids, are highly prevalent. Here, we investigated the effect of phosphatidylserine (PS)-containing liposomes, which bind to C2 domain with high affinity and specificity, upon the immunogenicity of rFVIII. Circular dichroism studies showed that PS-containing liposomes interfered with aggregation of rFVIII. Immunogenicity of free- versus liposomal-rFVIII was evaluated in a murine model of hemophilia A. Animals treated with s.c. injections of liposomal-rFVIII had lower total- and inhibitory titers, compared to animals treated with rFVIII alone. Antigen processing by proteolytic enzymes was reduced in the presence of liposomes. Animals treated with s.c. injections of liposomal-rFVIII showed a significant increase in rFVIII plasma concentration compared to animals that received rFVIII alone. Based on these studies, we hypothesize that specific molecular interactions between PS-containing bilayers and rFVIII may provide a basis for designing lipidic complexes that improve the stability, reduce the immunogenicity of rFVIII formulations, and permit administration by s.c. route.
2007 Wiley-Liss, Inc
Figures




References
-
- Foster PA, Zimmerman TS. Factor VIII structure and function. Blood Rev. 1989;3:180–191. - PubMed
-
- Fay PJ. Factor VIII structure and function. Thromb Haemost. 1993;70:63–67. - PubMed
-
- Larner AJ. The molecular pathology of haemophilia. Q J Med. 1987;63:473–491. - PubMed
-
- Klinge J, Ananyeva NM, Hauser CA, Saenko EL. Hemophilia A—from basic science to clinical practice. Semin Thromb Hemost. 2002;28:309–322. - PubMed
-
- Fijnvandraat K, Bril WS, Voorberg J. Immunobiology of inhibitor development in hemophilia A. Semin Thromb Hemost. 2003;29:61–68. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

