A stress-induced rice (Oryza sativa L.) beta-glucosidase represents a new subfamily of glycosyl hydrolase family 5 containing a fascin-like domain
- PMID: 17705786
- PMCID: PMC2267349
- DOI: 10.1042/BJ20070734
A stress-induced rice (Oryza sativa L.) beta-glucosidase represents a new subfamily of glycosyl hydrolase family 5 containing a fascin-like domain
Abstract
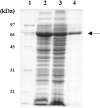
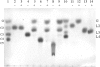
GH5BG, the cDNA for a stress-induced GH5 (glycosyl hydrolase family 5) beta-glucosidase, was cloned from rice (Oryza sativa L.) seedlings. The GH5BG cDNA encodes a 510-amino-acid precursor protein that comprises 19 amino acids of prepeptide and 491 amino acids of mature protein. The protein was predicted to be extracellular. The mature protein is a member of a plant-specific subgroup of the GH5 exoglucanase subfamily that contains two major domains, a beta-1,3-exoglucanase-like domain and a fascin-like domain that is not commonly found in plant enzymes. The GH5BG mRNA is highly expressed in the shoot during germination and in leaf sheaths of mature plants. The GH5BG was up-regulated in response to salt stress, submergence stress, methyl jasmonate and abscisic acid in rice seedlings. A GUS (glucuronidase) reporter tagged at the C-terminus of GH5BG was found to be secreted to the apoplast when expressed in onion (Allium cepa) cells. A thioredoxin fusion protein produced from the GH5BG cDNA in Escherichia coli hydrolysed various pNP (p-nitrophenyl) glycosides, including beta-D-glucoside, alpha-L-arabinoside, beta-D-fucoside, beta-D-galactoside, beta-D-xyloside and beta-D-cellobioside, as well as beta-(1,4)-linked glucose oligosaccharides and beta-(1,3)-linked disaccharide (laminaribiose). The catalytic efficiency (kcat/K(m)) for hydrolysis of beta-(1,4)-linked oligosaccharides by the enzyme remained constant as the DP (degree of polymerization) increased from 3 to 5. This substrate specificity is significantly different from fungal GH5 exoglucanases, such as the exo-beta-(1,3)-glucanase of the yeast Candida albicans, which may correlate with a marked reduction in a loop that makes up the active-site wall in the Candida enzyme.
Figures


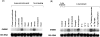

References
-
- Henrissat B., Davies G. T. Structural and sequence-based classification of glycoside hydrolases. Curr. Opin. Struct. Biol. 1997;7:637–644. - PubMed
-
- Jenkins J., Leggio L. L., Harris G., Pickersgill R. β-Glucosidase, β-galactosidase, family A cellulases, family F xylanases and two barley glycanases form a superfamily of enzymes with 8-fold β/α architecture and with two conserved glutamates near the carboxy-terminal ends of β-strands four and seven. FEBS Lett. 1995;362:281–285. - PubMed
-
- Coutinho P. M., Henrissat B. Carbohydrate-active enzymes: an integrated database approach. In: Gilbert H. J., Davies G., Henrissat B., Svensson B., editors. Recent Advances in Carbohydrate Bioengineering. Cambridge: Royal Society of Chemistry; 1999. pp. 3–12.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

