Inflammation and breast cancer. Balancing immune response: crosstalk between adaptive and innate immune cells during breast cancer progression
- PMID: 17705880
- PMCID: PMC2206719
- DOI: 10.1186/bcr1746
Inflammation and breast cancer. Balancing immune response: crosstalk between adaptive and innate immune cells during breast cancer progression
Abstract
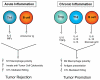
Recent insights into the molecular and cellular mechanisms underlying cancer development have revealed that immune cells functionally regulate epithelial cancer development and progression. Moreover, accumulated clinical and experimental data indicate that the outcome of an immune response toward an evolving breast neoplasm is largely determined by the type of immune response elicited. Acute tumor-directed immune responses involving cytolytic T lymphocytes appear to protect against tumor development, whereas immune responses involving chronic activation of humoral immunity, infiltration by Th2 cells, and protumor-polarized innate inflammatory cells result in the promotion of tumor development and disease progression. Herein we review this body of literature and summarize important new findings revealing the paradoxical role of innate and adaptive leukocytes as regulators of breast carcinogenesis.
Figures



References
-
- American Cancer Society . Cancer Facts & Figures 2007. Alanta, GA: American Cancer Society; 2007.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

