A continuous fluorometric assay for the assessment of MazF ribonuclease activity
- PMID: 17706586
- PMCID: PMC2443740
- DOI: 10.1016/j.ab.2007.07.017
A continuous fluorometric assay for the assessment of MazF ribonuclease activity
Abstract
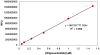
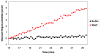
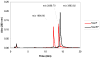
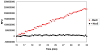
Plasmids maintain themselves in their bacterial host through several different mechanisms, one of which involves the synthesis of plasmid-encoded toxin and antitoxin proteins. When the plasmid is present, the antitoxin binds to and neutralizes the toxin. If a plasmid-free daughter cell arises, however, the labile antitoxin is degraded (and not replenished) and the toxin kills the cell from within. These toxin-antitoxin (TA) systems thereby function as postsegregational killing systems, and the disruption of the TA interaction represents an intriguing antibacterial strategy. It was recently discovered that the genes for one particular TA system, MazEF, are ubiquitous on plasmids isolated from clinical vancomycin-resistant enterococci (VRE) strains. Thus, it appears that small molecule disruptors of the MazEF interaction have potential as antibacterial agents. The MazF toxin protein is known to be a ribonuclease. Unfortunately, traditional methods for the assessment of MazF activity rely on the use of radiolabeled substrates followed by analysis with polyacrylamide gel electrophoresis. This article describes a simple and convenient continuous assay for the assessment of MazF activity. The assay uses an oligonucleotide with a fluorophore on the 5' end and a quencher on the 3' end, and processing of this substrate by MazF results in a large increase in the fluorescence signal. Through this assay, we have for the first time determined K(M) and V(max) values for this enzyme and have also found that MazF is not inhibited by standard ribonuclease inhibitors. This assay will be useful to those interested in the biochemistry of the MazF family of toxins and the disruption of MazE/MazF.
Figures





Similar articles
-
Toxin-antitoxin systems are ubiquitous and plasmid-encoded in vancomycin-resistant enterococci.Proc Natl Acad Sci U S A. 2007 Jan 2;104(1):311-6. doi: 10.1073/pnas.0601168104. Epub 2006 Dec 26. Proc Natl Acad Sci U S A. 2007. PMID: 17190821 Free PMC article.
-
Long Dynamic β1-β2 Loops in M. tb MazF Toxins Affect the Interaction Modes and Strengths of the Toxin-Antitoxin Pairs.Int J Mol Sci. 2024 Sep 5;25(17):9630. doi: 10.3390/ijms25179630. Int J Mol Sci. 2024. PMID: 39273577 Free PMC article.
-
Autoregulation of mazEF expression underlies growth heterogeneity in bacterial populations.Nucleic Acids Res. 2018 Apr 6;46(6):2918-2931. doi: 10.1093/nar/gky079. Nucleic Acids Res. 2018. PMID: 29432616 Free PMC article.
-
Distressing bacteria: structure of a prokaryotic detox program.Mol Cell. 2003 Apr;11(4):848-50. doi: 10.1016/s1097-2765(03)00146-1. Mol Cell. 2003. PMID: 12718870 Review.
-
Ribonucleases in bacterial toxin-antitoxin systems.Biochim Biophys Acta. 2013 Jun-Jul;1829(6-7):523-31. doi: 10.1016/j.bbagrm.2013.02.007. Epub 2013 Feb 21. Biochim Biophys Acta. 2013. PMID: 23454553 Review.
Cited by
-
Nitrosomonas europaea MazF Specifically Recognises the UGG Motif and Promotes Selective RNA Degradation.Front Microbiol. 2018 Oct 8;9:2386. doi: 10.3389/fmicb.2018.02386. eCollection 2018. Front Microbiol. 2018. PMID: 30349517 Free PMC article.
-
Exposing plasmids as the Achilles' heel of drug-resistant bacteria.Curr Opin Chem Biol. 2008 Aug;12(4):389-99. doi: 10.1016/j.cbpa.2008.06.015. Epub 2008 Jul 14. Curr Opin Chem Biol. 2008. PMID: 18625335 Free PMC article. Review.
-
The MazEF Toxin-Antitoxin System Alters the β-Lactam Susceptibility of Staphylococcus aureus.PLoS One. 2015 May 12;10(5):e0126118. doi: 10.1371/journal.pone.0126118. eCollection 2015. PLoS One. 2015. PMID: 25965381 Free PMC article.
-
Determination of ribonuclease sequence-specificity using Pentaprobes and mass spectrometry.RNA. 2012 Jun;18(6):1267-78. doi: 10.1261/rna.031229.111. Epub 2012 Apr 26. RNA. 2012. PMID: 22539524 Free PMC article.
-
Use of a fluorescence assay to determine relative affinities of semisynthetic aminoglycosides to small RNAs representing bacterial and mitochondrial A sites.Bioorg Med Chem. 2019 Nov 15;27(22):115121. doi: 10.1016/j.bmc.2019.115121. Epub 2019 Sep 13. Bioorg Med Chem. 2019. PMID: 31610941 Free PMC article.
References
-
- Zielenkiewicz U, Ceglowski P. Mechanisms of plasmid stable maintenance with special focus on plasmid addiction systems. Acta Biochim Pol. 2001;4:1003–1023. - PubMed
-
- Gerdes K, Christensen SK, Lobner-Olesen A. Prokaryotic toxin-antitoxin stress response loci. Nat Rev Microbiol. 2005;5:371–382. - PubMed
-
- Hayes F. Toxins-antitoxins: plasmid maintenance, programmed cell death, and cell cycle arrest. Science. 2003;5639:1496–1499. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

