The receptor protein tyrosine phosphatase (RPTP)beta/zeta is expressed in different subtypes of human breast cancer
- PMID: 17706593
- PMCID: PMC2084077
- DOI: 10.1016/j.bbrc.2007.06.050
The receptor protein tyrosine phosphatase (RPTP)beta/zeta is expressed in different subtypes of human breast cancer
Abstract
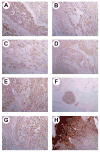
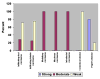
Increasing evidence suggests mutations in human breast cancer cells that induce inappropriate expression of the 18-kDa cytokine pleiotrophin (PTN, Ptn) initiate progression of breast cancers to a more malignant phenotype. Pleiotrophin signals through inactivating its receptor, the receptor protein tyrosine phosphatase (RPTP)beta/zeta, leading to increased tyrosine phosphorylation of different substrate proteins of RPTPbeta/zeta, including beta-catenin, beta-adducin, Fyn, GIT1/Cat-1, and P190RhoGAP. PTN signaling thus has wide impact on different important cellular systems. Recently, PTN was found to activate anaplastic lymphoma kinase (ALK) through the PTN/RPTPbeta/zeta signaling pathway; this discovery potentially is very important, since constitutive ALK activity of nucleophosmin (NPM)-ALK fusion protein is causative of anaplastic large cell lymphomas, and, activated ALK is found in other malignant cancers. Recently ALK was identified in each of 63 human breast cancers from 22 subjects. We now demonstrate that RPTPbeta/zeta is expressed in each of these same 63 human breast cancers that previously were found to express ALK and in 10 additional samples of human breast cancer. RPTPbeta/zeta furthermore was localized not only in its normal association with the cell membrane but also scattered in cytoplasm and in nuclei in different breast cancer cells and, in the case of infiltrating ductal carcinomas, the distribution of RPTPbeta/zeta changes as the breast cancer become more malignant. The data suggest that the PTN/RPTPbeta/zeta signaling pathway may be constitutively activated and potentially function to constitutively activate ALK in human breast cancer.
Figures


Similar articles
-
Anaplastic lymphoma kinase is expressed in different subtypes of human breast cancer.Biochem Biophys Res Commun. 2007 Jun 29;358(2):399-403. doi: 10.1016/j.bbrc.2007.04.137. Epub 2007 Apr 30. Biochem Biophys Res Commun. 2007. PMID: 17490616 Free PMC article.
-
Anaplastic lymphoma kinase is activated through the pleiotrophin/receptor protein-tyrosine phosphatase beta/zeta signaling pathway: an alternative mechanism of receptor tyrosine kinase activation.J Biol Chem. 2007 Sep 28;282(39):28683-28690. doi: 10.1074/jbc.M704505200. Epub 2007 Aug 6. J Biol Chem. 2007. PMID: 17681947
-
Fyn is a downstream target of the pleiotrophin/receptor protein tyrosine phosphatase beta/zeta-signaling pathway: regulation of tyrosine phosphorylation of Fyn by pleiotrophin.Biochem Biophys Res Commun. 2005 Jul 8;332(3):664-9. doi: 10.1016/j.bbrc.2005.05.007. Biochem Biophys Res Commun. 2005. PMID: 15925565
-
Anaplastic lymphoma kinase: "Ligand Independent Activation" mediated by the PTN/RPTPβ/ζ signaling pathway.Biochim Biophys Acta. 2013 Oct;1834(10):2219-23. doi: 10.1016/j.bbapap.2013.06.004. Epub 2013 Jun 15. Biochim Biophys Acta. 2013. PMID: 23777859 Review.
-
Pleiotrophin, a multifunctional tumor promoter through induction of tumor angiogenesis, remodeling of the tumor microenvironment, and activation of stromal fibroblasts.Cell Cycle. 2007 Dec 1;6(23):2877-83. doi: 10.4161/cc.6.23.5090. Cell Cycle. 2007. PMID: 18156802 Review.
Cited by
-
Analysis of the Cerebrospinal Fluid Proteome in Alzheimer's Disease.PLoS One. 2016 Mar 7;11(3):e0150672. doi: 10.1371/journal.pone.0150672. eCollection 2016. PLoS One. 2016. PMID: 26950848 Free PMC article.
-
PTN signaling: Components and mechanistic insights in human ovarian cancer.Mol Carcinog. 2015 Dec;54(12):1772-85. doi: 10.1002/mc.22249. Epub 2014 Nov 21. Mol Carcinog. 2015. PMID: 25418856 Free PMC article.
-
Pleiotrophin (PTN) expression and function and in the mouse mammary gland and mammary epithelial cells.PLoS One. 2012;7(10):e47876. doi: 10.1371/journal.pone.0047876. Epub 2012 Oct 15. PLoS One. 2012. PMID: 23077670 Free PMC article.
-
The role of pleiotrophin and beta-catenin in fetal lung development.Respir Res. 2010 Jun 18;11(1):80. doi: 10.1186/1465-9921-11-80. Respir Res. 2010. PMID: 20565841 Free PMC article. Review.
-
Anaplastic lymphoma kinase: role in cancer pathogenesis and small-molecule inhibitor development for therapy.Expert Rev Anticancer Ther. 2009 Mar;9(3):331-56. doi: 10.1586/14737140.9.3.331. Expert Rev Anticancer Ther. 2009. PMID: 19275511 Free PMC article. Review.
References
-
- Li YS, Milner PG, Chauhan AK, Watson MA, Hoffman RM, Kodner CM, Milbrandt J, Deuel TF. Cloning and expression of a developmentally regulated protein that induces mitogenic and neurite outgrowth activity. Science. 1990;250:1690–1694. - PubMed
-
- Deuel TF, Zhang N, Yeh HJ, Silos-Santiago I, Wang ZY. Pleiotrophin: a cytokine with diverse functions and a novel signaling pathway. Arch Biochem Biophys. 2002;397:162–171. - PubMed
-
- Vacherot F, Caruelle D, Chopin D, Gil-Diez S, Barritault D, Caruelle JP, Courty J. Involvement of heparin affin regulatory peptide in human prostate cancer. Prostate. 1999;38:126–136. - PubMed
-
- Soulie P, Heroult M, Bernard I, Kerros ME, Milhiet PE, Delbe J, Barritault D, Caruelle D, Courty J. Immunoassay for measuring the heparin-binding growth factors HARP and MK in biological fluids. J Immunoassay Immunochem. 2002;23:33–48. - PubMed
-
- Zhang L, Mabuchi T, Satoh E, Maeda S, Nukui H, Naganuma H. Overexpression of heparin-binding growth-associated molecule in malignant glioma cells. Neurol Med Chir (Tokyo) 2004;44:637–643. discussion 644–635. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

